Diisocyanate composition for optical lens and preparation method thereof
A technology of diisocyanate and xylylene diisocyanate, which is applied in the preparation of isocyanic acid derivatives, amino compounds, organic compounds, etc., can solve the problems of reduced yield, difficult application, high toxicity, etc., and achieve improved Yield effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1-1
[0186] Example 1-1: Preparation of diisocyanate composition
[0187] step 1)
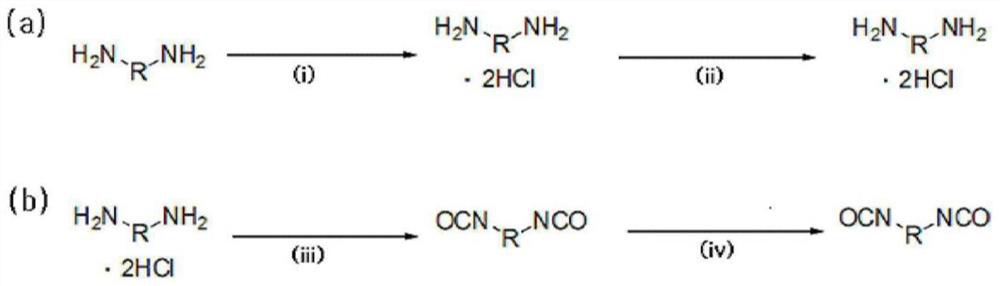
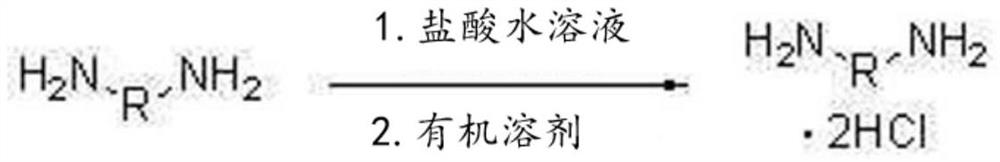
[0188]1,009.4 g (9.46 mol) of an aqueous hydrochloric acid solution having a concentration of 35% by weight was charged into the reactor, and then the internal temperature of the reactor was lowered to 15° C. with stirring. While maintaining the temperature of the reactor at 60° C., 600.0 g (4.4 moles) of m-XDA were introduced for 1 hour. Here, the amount of aqueous hydrochloric acid introduced corresponds to 2.15 moles of HCl per 1 mole of m-XDA. After the introduction was completed, the internal temperature of the reactor was lowered to 10° C., and it was stirred for 1 hour. Thereafter, 1,320.0 g of tetrahydrofuran (THF) was introduced, and the internal temperature of the reactor was lowered to -5° C., followed by stirring for 1 hour. After the reaction, the diamine hydrochloride composition containing m-XDA·2HCl was separated by vacuum filtration using a filter, and the filtered tetrahydrofura...
example 2-1
[0215] Example 2-1: Preparation of diisocyanate composition
[0216] step 1)
[0217] 1,009.4 g (9.46 mol) of an aqueous hydrochloric acid solution having a concentration of 35% by weight was charged into the reactor, and then the internal temperature of the reactor was lowered to 15° C. with stirring. While maintaining the temperature of the reactor at 60° C., 600.0 g (4.4 moles) of m-XDA were introduced for 1 hour. Here, the amount of aqueous hydrochloric acid introduced corresponds to 2.15 moles of HCl per 1 mole of m-XDA. After the introduction was completed, the internal temperature of the reactor was lowered to 10° C., and it was stirred for 1 hour. Thereafter, 1,320.0 g of tetrahydrofuran (THF) was introduced, and the internal temperature of the reactor was lowered to -5° C., followed by stirring for 1 hour. After the reaction, the diamine hydrochloride composition containing m-XDA·2HCl was separated by vacuum filtration using a filter, and the filtered tetrahydrofur...
example 2-2 to 2-5 and comparative example 2-1 to 2-3
[0221] The procedure of step (1) of Example 2-1 was repeated, except that the amount of aqueous hydrochloric acid introduced was changed as shown in Table 3 below to obtain the diamine hydrochloride composition, and the procedure of step (2) according to Example 2-1 was obtained by Diamine Hydrochloride Compositions Diisocyanate compositions are prepared.
[0222] The pHs of the diamine hydrochloride compositions obtained in step (1) of Examples and Comparative Examples respectively when dissolved in water at a concentration of 10% by weight are summarized in Table 3 below.
[0223] [table 3]
[0224]
[0225] As can be seen from the table above, the pH of the diamine hydrochloride salt composition can be varied by adjusting the amount of aqueous hydrochloric acid introduced.
[0226] Preparation of Optical Lenses
[0227] The m- XDI composition, the dibutyl tin dichloride of 0.01 weight part and the phosphate ester release agent (ZELEC) of 0.1 weight part TM UN Stepan...
PUM
| Property | Measurement | Unit |
|---|---|---|
| haze | aaaaa | aaaaa |
| transmittivity | aaaaa | aaaaa |
| haze | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com