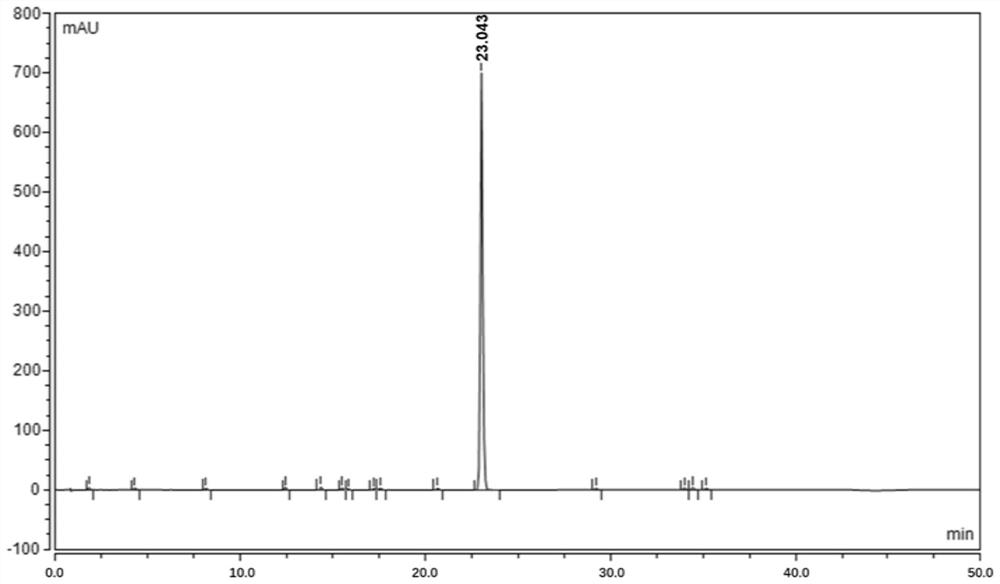
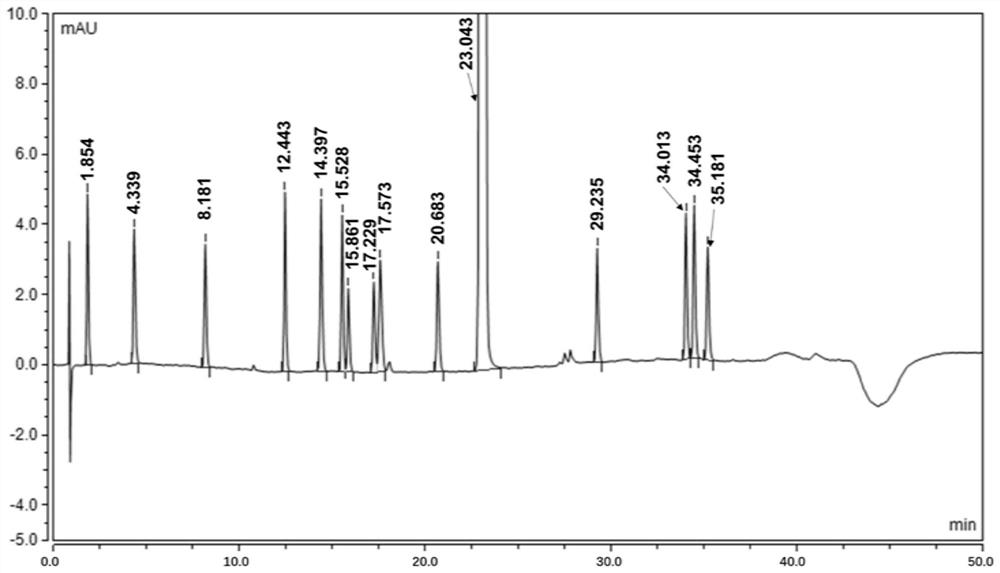
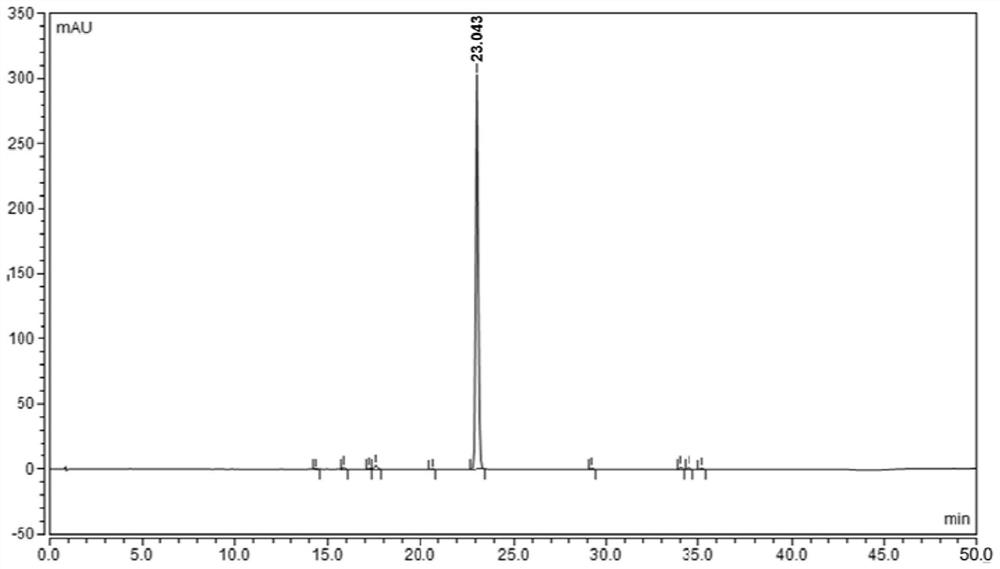
An analytical method for determining rivaroxaban and its impurities
An analytical method and rivaroxaban technology, applied in the field of analytical chemistry, can solve the problems of blockage of chromatographic column, high cost of buffer salt solution, strict operation requirements, etc., and achieve the effect of eliminating interference, strong specificity and improving durability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] An analytical method for determining rivaroxaban and its impurities
[0057] 1.1 Preparation of impurity stock solution:
[0058] Take impurity L, impurity A, impurity O, impurity B, impurity C, impurity I, impurity M, impurity K, impurity N, impurity D, impurity E, impurity F, impurity RC18, impurity G, and use solvent [acetonitrile- Water (20:80)] ultrasonically dissolved and quantitatively diluted to a solution of about 20ug / ml.
[0059] 1.2 Preparation of system suitability solution:
[0060] Take about 10mg of rivaroxaban, weigh it accurately, put it in a 50ml volumetric flask, add 10ml of acetonitrile and ultrasonically dissolve it completely, add 1ml of each impurity stock solution, dilute to the mark with a solvent, and shake well.
[0061] 1.3 Preparation of the test solution:
[0062] Take about 85 mg of fine powder of rivaroxaban tablets, weigh it accurately, put it in a 50ml measuring bottle, add 10ml of acetonitrile and sonicate for 15 minutes to dissolv...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com