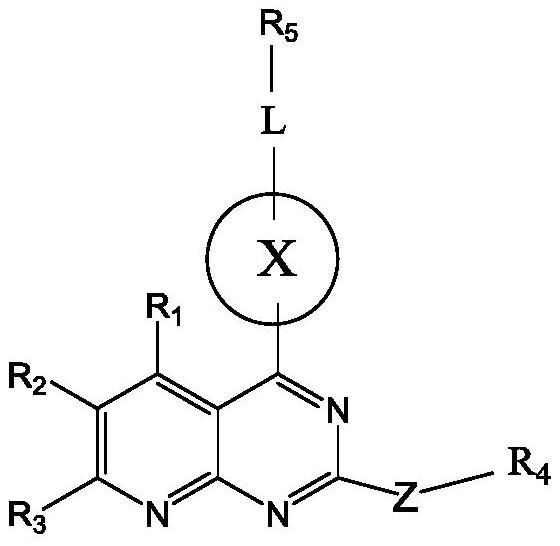
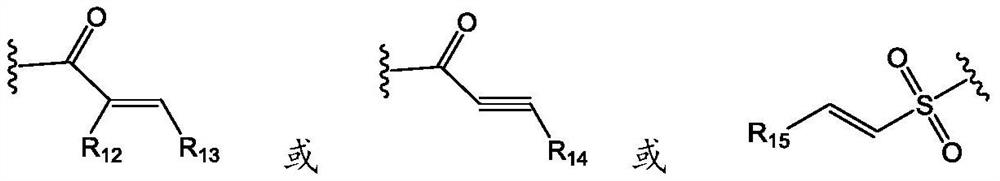
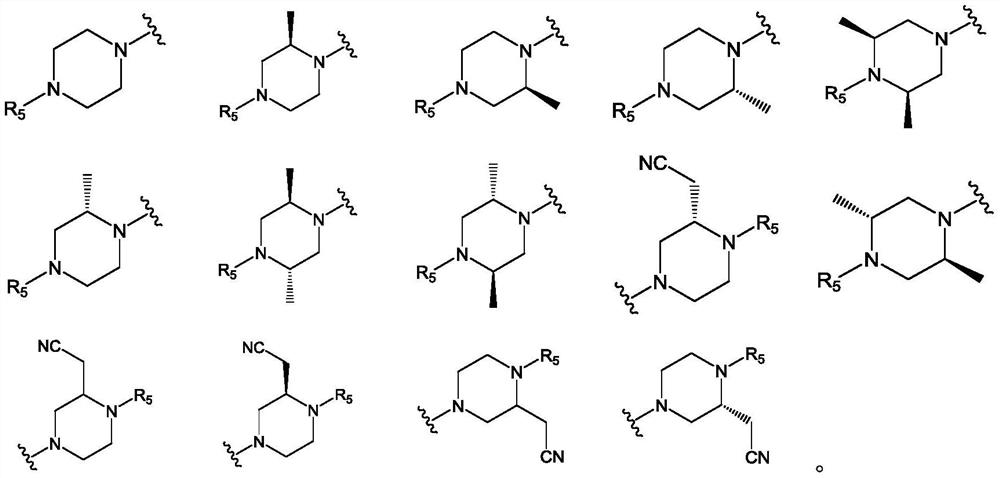
Pyridopyrimidine KRAS G12C mutant protein inhibitor
A technology of selecting and compounding compounds, applied in the field of medicine, can solve the problem of insufficient high-efficiency and high-safety K-RASG12C inhibitors, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0082]
[0083] Compound (S)-1-(4-(7-(naphthalen-1-yl)-2-((1-methylpyrrolidin-2-yl)methoxy)pyrido[2,3-d]pyrimidine The synthesis of -4-yl)piperazin-1-yl)prop-2-en-1-one refers to the general reaction scheme (A) and (B); MS (ESI) m / z509.26[M+H] + .
[0084] The synthetic route and specific method are as follows:
[0085]
[0086] first step
[0087]Compound A (500.0 mg, 2.9 mmol, 1.00 eq) was dissolved in 5 mL of toluene, and oxalyl chloride (443.8 mg, 3.5 mmol, 1.20 eq) was added under nitrogen protection. The mixture was stirred at 110°C for 15 hours. Cool to room temperature, filter, wash the filter residue with toluene (5 mL×2), and dry. The crude compound B (528 mg, light yellow solid) was obtained, and the product was directly carried out to the next reaction without purification.
[0088] second step
[0089] Crude compound B (100.0mg, 0.5mmol, 1.00eq) was dissolved in 0.6mL of toluene, and N,N-diisopropylethylamine (196.3mg, 1.5mmol, 3.00eq) was added under ...
Embodiment 2
[0101]
[0102] Compound 1-((S)-3-methyl-4-(7-(naphthalene-1-yl)-2-(((S)-1-methylpyrrolidin-2-yl)methoxy)pyridine [2,3-d]pyrimidin-4-yl)-3-methylpiperazin-1-yl)prop-2-en-1-one is synthesized according to the general reaction scheme (A) and (B); MS (ESI)m / z 523.28[M+H] + .
[0103] The synthetic route and specific method are as follows:
[0104]
[0105] first step
[0106] Compound A (100.0mg, 0.5mmol, 1.00eq) was dissolved in 0.6mL of toluene, and N,N-diisopropylethylamine (196.3mg, 1.5mmol, 3.00eq) was added under nitrogen protection. The mixture was stirred at 70°C for 30 minutes, phosphorus oxychloride (232.8mg, 1.5mmol, 3.00eq) was added, and stirred under reflux at 100°C for 2.5 hours. After the reaction was complete, the solvent was spin-dried under reduced pressure, added water (1 mL), extracted with ethyl acetate (1 mL×3), the combined organic phase was washed with saturated sodium chloride solution (1 mL), dried over anhydrous sodium sulfate, filtered, and ...
Embodiment 3
[0116]
[0117] Compound 1-(4-(2-(2-(dimethylamino)ethoxy)-7-(3-hydroxynaphthalen-1-yl)pyrido[2,3-d]pyrimidin-4-yl) The synthesis of piperazin-1-yl)prop-2-en-1-one refers to the general reaction scheme (A) and (B); MS (ESI) m / z 499.24 [M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com