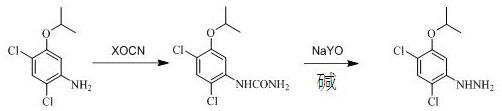
Preparation method of oxadiazon intermediate 2, 4-dichloro-5-isopropoxyphenylhydrazine
A technology for isopropoxyphenylhydrazine and intermediates, which is applied in the field of preparing 2,4-dichloro-5-isopropoxyphenylhydrazine, can solve the problems of difficult waste water treatment, expensive raw materials, difficult recovery and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Add 200 g of 2,4-dichloro-5-isopropoxyaniline toluene solution with an internal standard content of 35% into a 500 mL four-neck flask with a condenser tube inserted, slowly pour 42 g of 30% hydrochloric acid at room temperature, and continue After stirring for 0.5h, add 33.5g of potassium cyanate, then slowly raise the temperature to 100-105°C, reflux for 2h, and trace the raw material 2,4-dichloro-5-isopropoxyaniline by gas chromatography <1%, the reaction is over , cooling to obtain an intermediate (phenyl urea), to be added sodium hypochlorite alkali solution.
[0031] When the temperature drops to 20-25°C, start to drop 372g of sodium hypochlorite alkali solution mixed in advance (the molar ratio of urea, sodium hypochlorite, and alkali is 1:1.25:2.6), and control the temperature of the whole process not to exceed 25°C. After completion, keep the reaction for 40 minutes, then slowly raise the temperature to 80-85°C, continue the heat preservation reaction for 1 hour...
Embodiment 2
[0034] The preparation of phenylurea (intermediate) is the same as in Example 1, and the second step of degradation and rearrangement process replaces sodium hypochlorite with sodium hypobromite to obtain 2,4-dichloro-5-isopropoxyphenylhydrazine with a purity of 98.8%. The two-step yield of 2,4-dichloro-5-isopropoxyaniline to phenylhydrazine is 92.69%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com