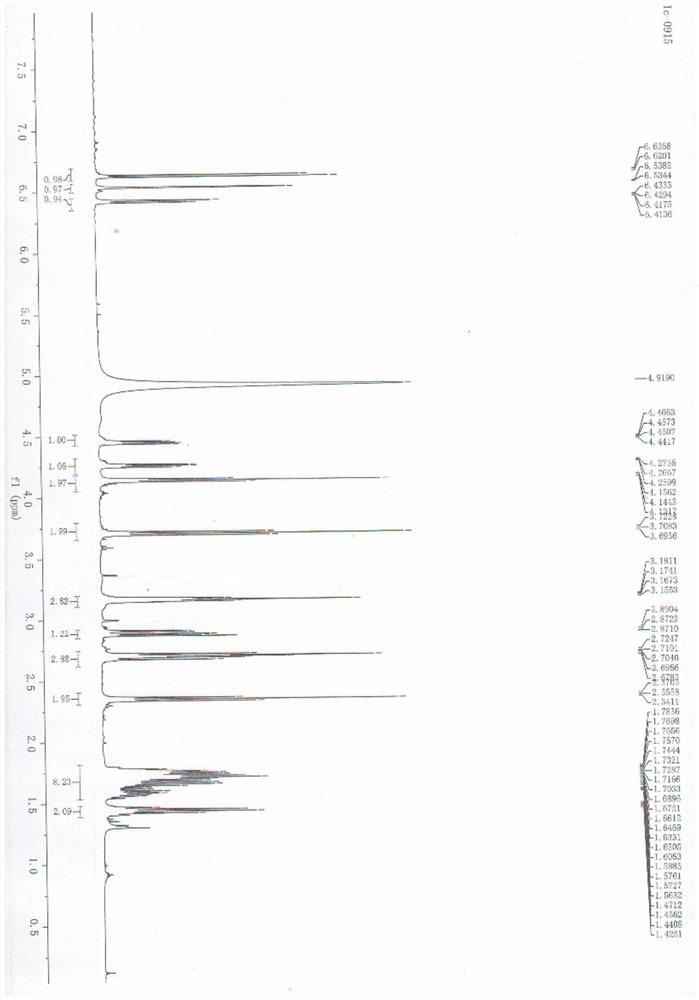
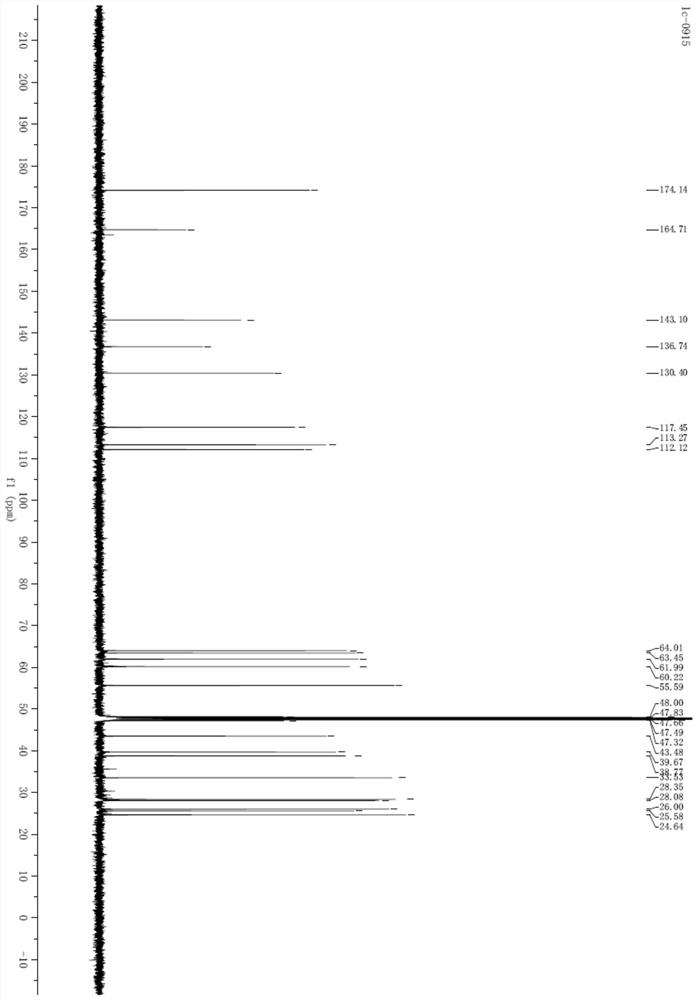
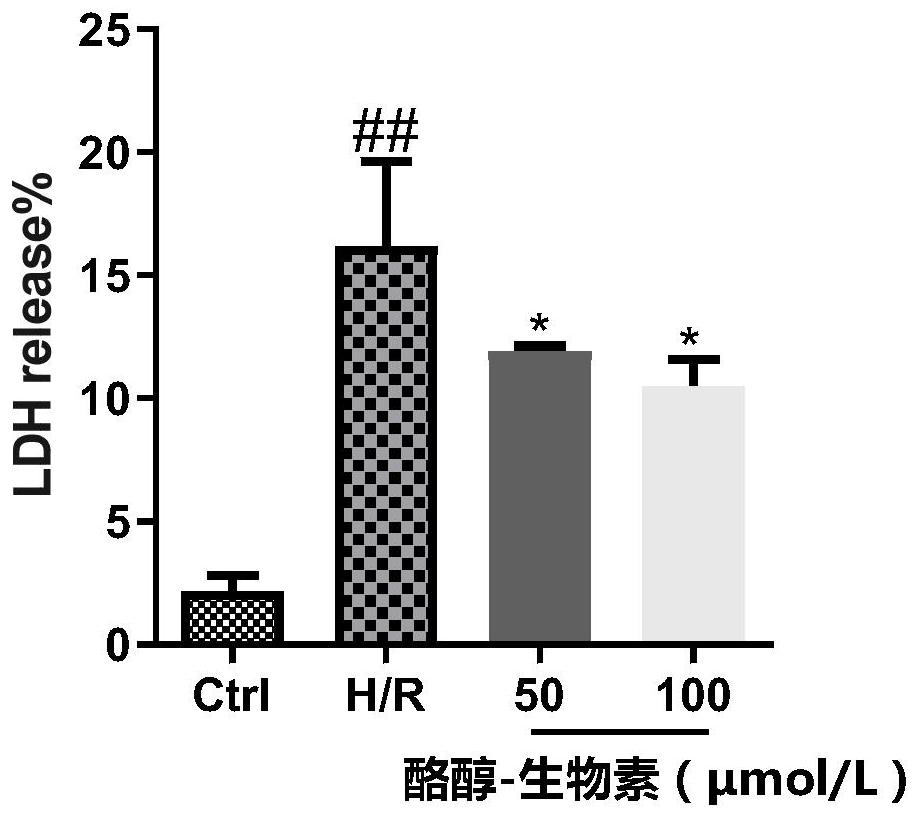
Tyrosol-biotin small molecular probe as well as preparation method and application thereof
A technology of small molecule probes and biotin, which is applied in the direction of drug combination, antidote, cardiovascular system diseases, etc., to achieve the effect of simple operation, mild reaction conditions and high selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Embodiment 1: the preparation of 2-nitrotyrosol (VII)
[0042]
[0043] In a 50 mL round bottom flask, add 934 mg of iodobenzene diacetoxy acetate, 1.3 g of zinc nitrate hexahydrate and 25 mL of acetonitrile, and add 2.0 g of 2-aminotyrosol (VI). The reaction solution was reacted at room temperature overnight, TLC detected that the raw materials were basically reacted completely, water was added, extracted with ethyl acetate, and the organic phase was concentrated to obtain 1.67 g of a tan solid with a yield of 63%.
Embodiment 2
[0044] Embodiment 2: the preparation of 2-nitrotyrosol (VII)
[0045] In a 50 mL round bottom flask, add 1.47 g of bis(tert-butylcarbonyloxy)iodobenzene, 1.3 g of zinc nitrate hexahydrate and 25 mL of acetonitrile, and add 2.0 g of tyrosol. The reaction solution was reacted at room temperature overnight, TLC detected that the raw materials were basically reacted completely, water was added, extracted with ethyl acetate, and the organic phase was concentrated to obtain 1.59 g of a brownish yellow solid with a yield of 60%.
Embodiment 3
[0046] Embodiment 3: the preparation of 2-nitrotyrosol (VII)
[0047] In a 50 mL round bottom flask, 1.25 g of [bis(trifluoroacetoxy)iodo]benzene, 1.3 g of zinc nitrate hexahydrate and 25 mL of acetonitrile were added, and 2.0 g of tyrosol was added. The reaction solution was reacted overnight at room temperature. TLC detected that the raw materials were basically reacted completely. Water was added, extracted with ethyl acetate, and the organic phase was concentrated to obtain 2.12 g of a brownish-yellow solid with a yield of 80%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com