Synthesis method of 1-phenylvinyl borate
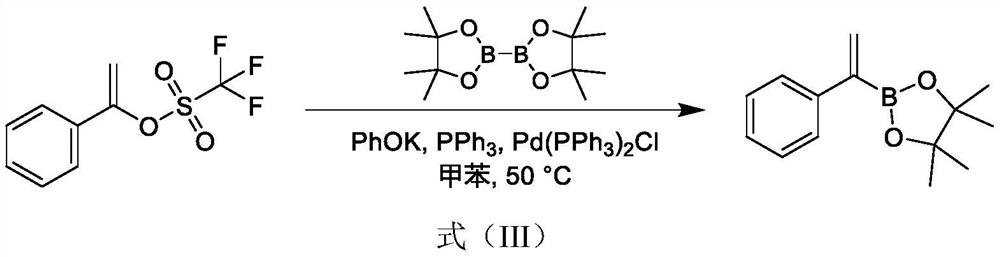
A technology of phenyl vinyl borate and synthesis method, which is applied in the field of synthesis of phenyl vinyl borate, can solve the problem of high price, increased cost, and difficulty in obtaining 1-phenyl vinyl trifluoromethanesulfonate problems such as good reaction selectivity, reduced reagent cost, and easy post-processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
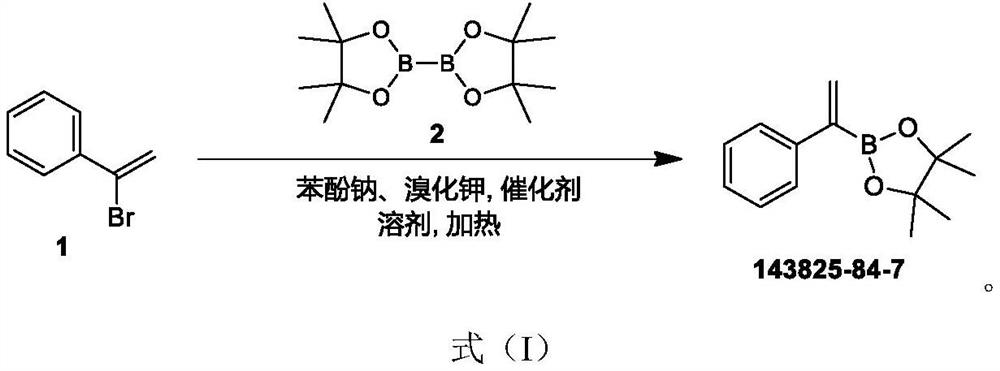
[0022] Sodium phenoxide PhONa (95.13g ,0.82mol), KBr (97.52g, 0.82mol), triphenylphosphine PPh 3 (4.31g, 16.44mmol) and dichlorobis(triphenylphosphine)palladium Pd(PPh 3 ) 2 Cl 2 (7.68 g, 10.96 mmol). Stir for 16 hours at 70°C under the protection of inert gas. TLC (petroleum ether:ethyl acetate=15:1) showed that starting material 1 ((1-bromovinyl)benzene) was consumed and new compounds were formed. After the reaction mixture was filtered, the filtrate was concentrated under reduced pressure to remove the solvent. The concentrated solution was diluted with water and extracted with ethyl acetate solvent, the extracted organic layer was washed with brine, Na 2 SO 4 Dry, filter and concentrate under reduced pressure to obtain a crude product. The crude product was then diluted with petroleum ether and ethyl acetate, stirred for several hours, filtered, and the filtrate was collected and concentrated to obtain a solid, which was recrystallized at 0°C with petroleum ether t...
Embodiment 1
[0023] The reaction formula of embodiment 1 is as follows:
[0024]
Embodiment 2
[0026] See Example 1 for details of the reaction conditions. After the reaction mixture was filtered, the filtrate was concentrated under reduced pressure to remove the solvent. The concentrated solution was diluted with water and extracted with ethyl acetate solvent, the extracted organic layer was washed with brine, Na 2 SO 4 Dry, filter and concentrate under reduced pressure to obtain a crude product. The crude product was then diluted with petroleum ether and ethyl acetate, stirred for several hours, filtered, and the filtrate was collected and concentrated to obtain a solid, which was recrystallized at -10°C with petroleum ether: ethyl acetate = 10:1 to obtain the final product as a yellow solid (66.92g, 0.291mol, 53.2% yield).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com