Method for conjugation of biomolecules and new use of gold donor for biomolecular complex formation
A technology of biomolecules and complexes, which is applied to the preparation methods of peptides, medical preparations of non-active ingredients, chemical instruments and methods, etc., and can solve problems such as toxicity of nanoparticles and obstacles to in vivo application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
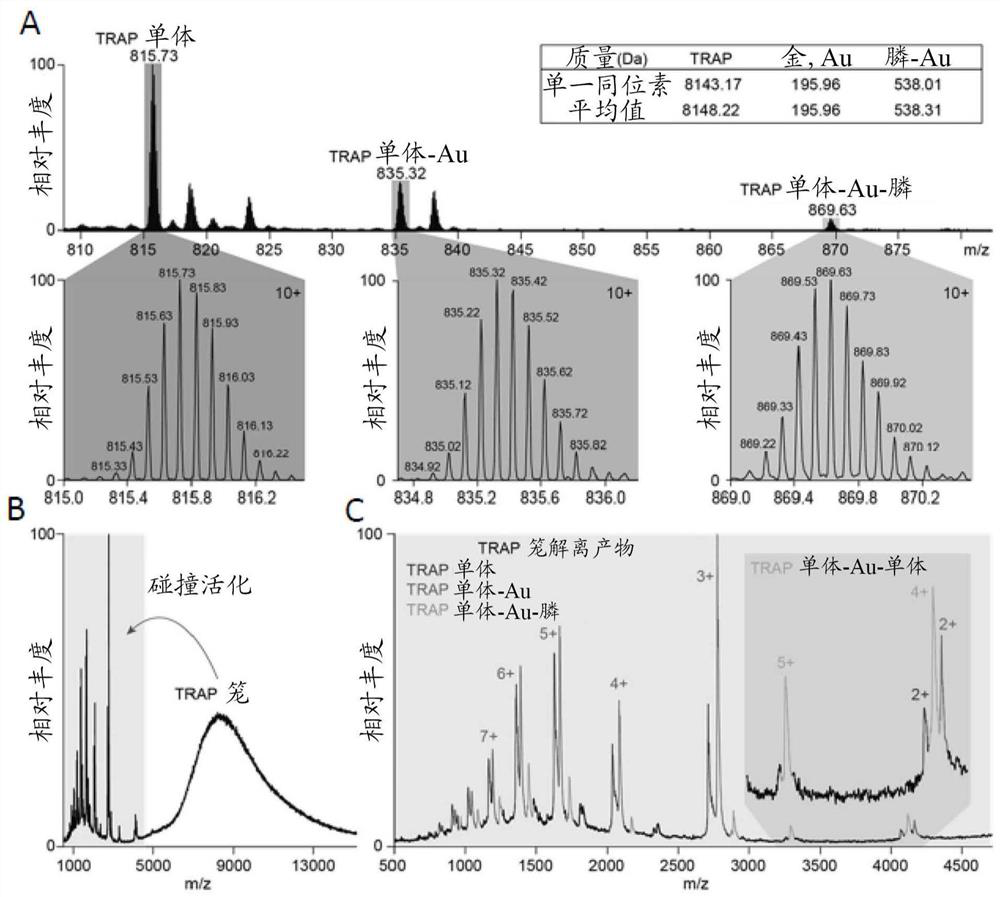
[0073] TRAP complex Preparation - Reaction with Au-TPPMS
[0074] (See figure 1 )
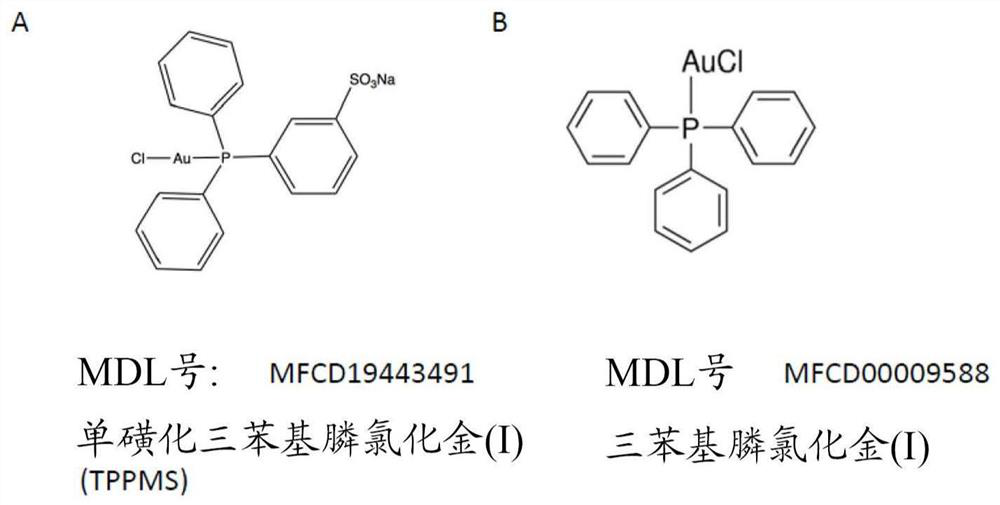
[0075] Gold compound:
[0076] [Diphenyl (3-sulfonate] phosphine] Chloride (I), sodium salt hydrate (Au-TPPMS, MDL MFCD19443491) is purchased from STREM CHEMICALS UK, LIMITED, and achieved by dissolving in water The desired concentration (usually 5 mm). The gold nanoparticles (GNP) used are a diphenyl group (mexylphenyl) phosphine having 1-3 nm inner diameter from StREM CHEMICALS UK (MDL MFCD 17018839).
[0077] TRAP preparation:
[0078] The protein used to successfully use Au-TPPMS is a TRAP protein having introduced cysteine. Expression and purification of TRAP containing 35 residual lysine (K) to cysteine and 64 residual argin (R) to serine (S) Additional mutation (called "TRAP-CS ") And the aforementioned TRAP-CS 11 (As described in detail above), significant changes are TCEP (three (2-carboxyethyl) phosphine) is not included in the cracking step. The final buffer is usually 20 mM Tris-HCl,...
Embodiment 2
[0083] Preparation of TRAP Composites - Reaction with Au (I) - Trobenzylphosphine
[0084] Trobhenylphosphine chloride (I) (Au-TPP, figure 1 B) A similar reaction instead of Au-TPPMS. The Au-TPP was dissolved in DMSO, reacted in 50 mM Tris, 150 mM NaCl, pH 7.9 and Au-TPP, and DMSO did not exceed 10% of the final volume, and the TRAP monomer was present at a concentration of about 1 mm. The ratio of Au-TPP and TRAP is 4: 2 to 4: 3. This also results in a TRAP cage formed from the same structure obtained in the Au-TPPMS reaction. The amount of the reagent is adjusted separately. The ratio and reaction conditions of the TRAP monomer / gold donor were the same as where Au-TPPMs was reacted as a gold donor.
[0085] Two embodiments having different halogen (triaryl phosphine) gold (I) gold donor reagents were carried out above. It shows a halogen (triaryl phosphine) gold (I) having a different aryl moiety (I) is adapted to form a complex according to the present invention.
Embodiment 3
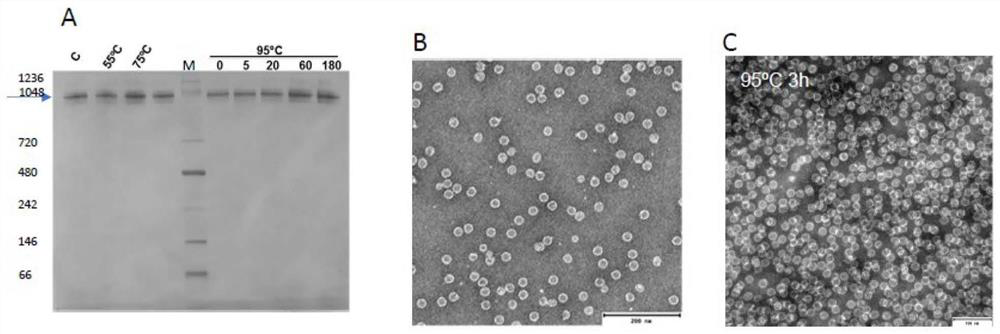
[0087] Use Cryo-EM to confirm the TRAP complex structure
[0088] The initial (low resolution) Cryo-EM structure of the Trap cage was obtained using a Cryo-EM single particle recombination technique for using a TRAP cage formed using GNP. 10,11 And the structure data is used as an initial model of the high resolution Cryo-EM structure formed in the TRAP cage formed in the reaction in the reaction in the reaction in the reaction in accordance with the present invention.
[0089] The structure of the TRAP cage was resolved using Cryo-EM to 3.9 angstroms. This is sufficient to display the arrangement of 24 TRAP loops and demonstrate that there is a connection density (specified as AU) between the crystallysite chains of the relative ring (see figure 2 ).
[0090] figure 2 Examples of the gold sewing reaction and the formation of the complex are exemplified. figure 2 A shows the structure of a single TRAP ring (PDB4V4F) that is displayed in two mutually orthogonal views. The mutat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com