Synthesis method of thiodicarb
A synthesis method and technology of thiodicarb, applied in the direction of organic chemistry, etc., can solve the problems of poor thermal storage stability, many by-products, shortened reaction time, etc. economic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] 1. Prepare materials
[0055] 295Kg of xylene is sucked into the synthesis kettle by vacuum, and the stirring is started;
[0056] Metering SCl 2 Liquid 141Kg, pumped into the high tank by vacuum;
[0057] Measure 104Kg of pyridine, and pump it into the synthesis kettle;
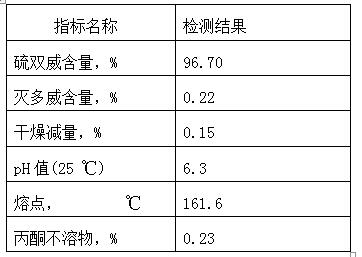
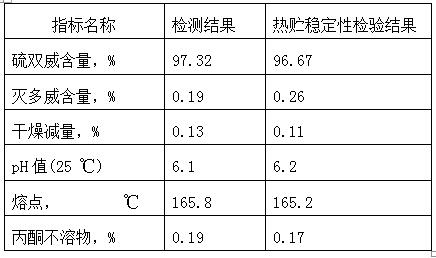
[0058] Measure methomyl 400Kg (content 97.3%), stand-by;
[0059] Measure 1.6Kg of catalyst trialkyl tertiary amine;
[0060] 2. Ligand synthesis
[0061] Put refrigerant liquid into the jacket of the synthetic kettle for use, cool down the temperature in the kettle to -4°C, and start adding SCl dropwise 2 , control the rate of addition, the reaction temperature was maintained at 3°C, and the addition time was controlled at 90 minutes. SCl 2 After the dropwise addition, continue to stir for 20 minutes;
[0062] 3. Thiodicarb synthesis
[0063] Drain the refrigerant in the jacket of the synthetic kettle, add 400Kg of methomyl and 1.6Kg of catalyst from the manhole, then stir for 20 minutes, fe...
Embodiment 2
[0079] 1. Prepare materials
[0080] 298Kg of xylene is sucked into the synthesis kettle by vacuum, and the stirring is started;
[0081] Metering SCl 2 Liquid 153Kg, pumped into the high tank by vacuum;
[0082] Measure 108Kg of pyridine, and pump it into the synthesis kettle;
[0083] Measure methomyl 400Kg (content 97.3%), stand-by;
[0084] Measure 2Kg of catalyst and set it aside.
[0085] 2. Ligand synthesis
[0086] Put refrigerant liquid into the jacket of the synthetic kettle for use, cool down the temperature in the kettle to -3°C, and start adding SCl dropwise 2 , control the rate of addition, the reaction temperature was maintained at 4 ° C, and the time of addition was controlled at 100 minutes. SCl 2 After the dropwise addition was complete, stirring was continued for 25 minutes.
[0087] 3. Thiodicarb synthesis
[0088] Drain the frozen liquid in the jacket of the synthetic kettle, add 400Kg of methomyl and 2Kg of catalyst from the manhole, then stir fo...
Embodiment 3
[0102] 1. Prepare materials
[0103] 370Kg of xylene is sucked into the synthesis kettle by vacuum, and the stirring is started;
[0104] Metering SCl 2 Liquid 163Kg, pumped into the high tank by vacuum;
[0105] Meter 120Kg of pyridine and pump it into the synthesis kettle;
[0106] Measure methomyl 450Kg (content 97.3%), stand-by;
[0107] Measure 2.5Kg of catalyst and set it aside.
[0108] 2. Ligand synthesis
[0109] Put refrigerant into the jacket of the synthetic kettle for use, cool down the temperature in the kettle to -2°C, and start adding SCl dropwise 2 , control the rate of addition, the reaction temperature was maintained at 5 ° C, and the time of addition was controlled at 110 minutes. SCl 2 After the dropwise addition was complete, stirring was continued for 20 minutes.
[0110] 3. Thiodicarb synthesis
[0111] Drain the frozen liquid in the jacket of the synthesis kettle, add 400Kg of methomyl and 2.5Kg of catalyst from the manhole, then stir for 25 min...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com