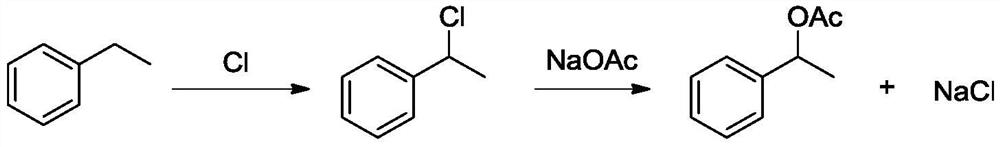
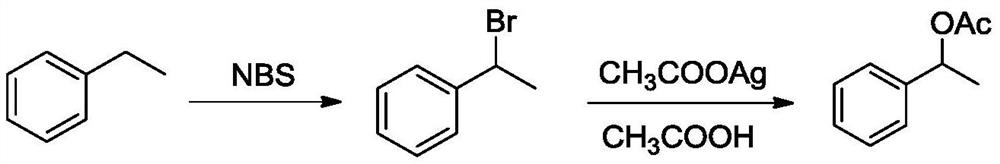
Method for synthesizing styrallyl acetate from acetophenone
A technology for styroyl acetate and acetophenone, which is applied in the field of synthesizing styroyl acetate, can solve problems such as inconvenience in large-scale production, easy polymerization of styrene, and potential safety hazards, and achieves the effects of novel synthetic route, good solubility, and easy operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
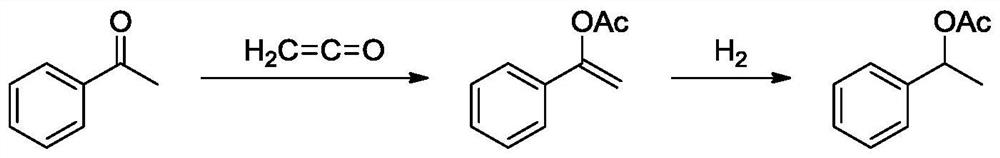
[0064] Vapor-phase esterification of acetophenone and ketene to styrene acetate:
[0065] Self-made 500mL esterification reaction kettle with stirring, the top of the reaction kettle is equipped with a serpentine condenser tube with a length of 40cm. Kettle, ketene is fed continuously. Add 242.73g of acetophenone and 0.97g of methanesulfonic acid into the esterification reaction kettle. The ketene gas is supplied from a self-made ketene reactor, and the gas outlet is inserted into the bottom of the reaction kettle. The feed rate is 0.60L / min. The stirring speed is 400r / min, and the esterification temperature is controlled at 65°C. The heat preservation reaction was carried out for 3 hours, and the sampling gas phase analysis showed that the conversion rate was 99%, and the selectivity was 99%. Stop feeding ketene, add 50g of saturated sodium bicarbonate aqueous solution to wash three times, add 50g of saturated brine to wash three times, dry at 50°C using anhydrous magnesium...
Embodiment 2
[0071] Vapor-phase esterification of acetophenone and ketene to styrene acetate:
[0072] Self-made 500mL esterification reaction kettle with stirring, the top of the reaction kettle is equipped with a serpentine condenser tube with a length of 40cm. Kettle, ketene is fed continuously. 242.73 g of acetophenone and 1.60 g of benzenesulfonic acid were added to the esterification reactor, and the ketene gas was supplied from a self-made ketene reactor, the gas outlet was inserted into the bottom of the reactor, and the feed rate was 0.60 L / min. The stirring speed is 400r / min, and the esterification temperature is controlled at 65°C. The heat preservation reaction was carried out for 3 hours, and the sampling gas phase analysis showed that the conversion rate was 98%, and the selectivity was 98%. Stop feeding ketene, add 50g of saturated sodium bicarbonate aqueous solution to wash three times, add 50g of saturated brine to wash three times, dry at 50°C using anhydrous magnesium ...
Embodiment 3
[0076] Vapor-phase esterification of acetophenone and ketene to styrene acetate:
[0077] Self-made 500mL esterification reaction kettle with stirring, the top of the reaction kettle is equipped with a serpentine condenser tube with a length of 40cm. Kettle, ketene is fed continuously. 242.73 g of acetophenone and 0.78 g of methanesulfonic acid were added to the esterification reactor, and the ketene gas was supplied from a self-made ketene reactor, the gas outlet was inserted into the bottom of the reactor, and the feed rate was 0.60 L / min. The stirring speed is 400r / min, and the esterification temperature is controlled at 65°C. Insulated reaction for 3 hours, sampling gas phase analysis, the conversion rate was 98%, and the selectivity was 97%. Stop feeding ketene, add 50g of saturated sodium bicarbonate aqueous solution to wash three times, add 50g of saturated brine to wash three times, dry at 50°C using anhydrous magnesium sulfate for 20min, distill under reduced pressu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com