Scylla paramamosain sensitizing protein Scy p 1 derivative and application thereof
A technology of simulating Scylla sinensis and derivatives is applied in the field of bioengineering, which can solve the problems such as the transformation of allergenic proteins to be studied, and achieve the effects of improving the safety index and reducing side effects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1 Molecular cloning of allergenic protein Scy p 1 derivatives
[0031] (1) Design and synthesis of primers
[0032] According to the amino acid sequence (SEQ ID NO: 1) of the derivative of Scy p 1, the gene sequence of Scy p 1 obtained from NCBI was deleted, and the upper part of the derivative of Scy p 1 was designed by Primer 5.0 software. Downstream primers (F, R). At the same time, Nde I and EcoR I restriction endonuclease sites were added on the basis of the upstream and downstream primers. In addition, the forward and reverse primers (F mutants , R mutants ), the primers used in the experiment were synthesized by Bosun Biotechnology Co., Ltd.
[0033] f 1 : 5'-CATATGATGAAGCTGGAGAAGGACA-3'; (SEQ ID NO: 3)
[0034] R 1 : 5'-TACGCTCGTCCTCCTCGAGGAGCTG-3'; (SEQ ID NO: 4)
[0035] f mutants : 5'-GAGGACGAGCGTATGCGTAAGGTGC-3'; (SEQ ID NO: 5)
[0036] R mutants : 5'-TACGCTCGTCCTCCTCGAGGAGCTG-3'. (SEQ ID NO: 6)
[0037] (2) Construction of pET-28a-Scy p...
Embodiment 2
[0052] Example 2 Preparation of wild-type Scy p 1 and Scy p 1 derivatives
[0053] (1) Induced expression of wild-type Scy p 1 and Scy p 1 derivatives
[0054] The strains of wild-type Scy p 1 and derivatives of Scy p 1 were cultured in 10 mL LB medium overnight. The next day, the above culture solution was transferred to 500 mL of LB culture solution at a ratio of 1:1000, and cultured at 37°C until OD 600 =0.6~0.8, add IPTG at a final concentration of 0.5mmol / L, continue induction at 37°C for 16h, then centrifuge (12000g, 15min) to collect the bacteria; resuspend in 20mL pre-cooled ultrasonic buffer (20mmol / L Tris-HCl, 0.2 mol / L NaCl, pH 8.0), the supernatant was collected after centrifugation (12000g, 30min), which was the crude enzyme solution.
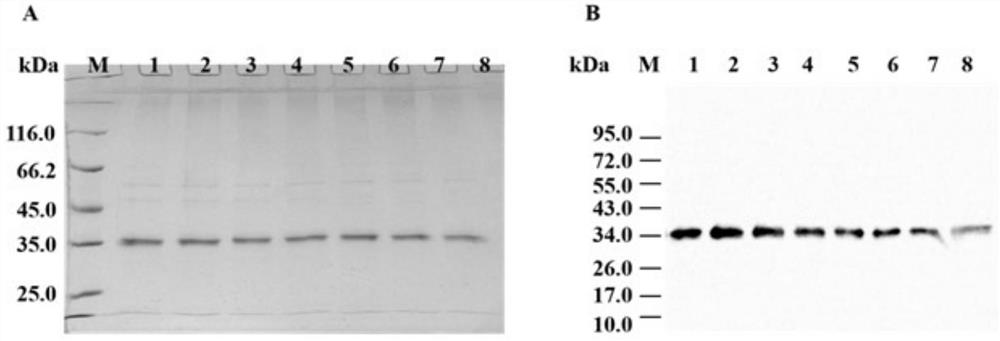
[0055] (2) Purification and identification of wild-type Scy p 1 and Scy p 1 derivatives
[0056] Load the crude enzyme solution in (1) at a speed of 1mL / min on the pre-balanced Ni 2+ - NTA affinity chromatography column (1.0 cm...
Embodiment 3
[0057] Example 3 Mast cell model detection of degranulation efficiency of wild-type Scy p 1 and Scy p 1 derivatives on LAD2 cells
[0058] Human mast cell LAD2 cells were used to establish a degranulation model, and 25 μL of wild-type Scy p 1 and Scy p 1 derivatives (final concentration 50 μg / mL) were added to LAD2 cells that had been sensitized by the serum of patients with crustacean allergy. The cells were stimulated by incubating for 1.0 h in a cell culture box, centrifuged, the supernatant was collected and the cells were lysed, and the wild-type Scy p 1 and Scy p 1 derivatives stimulated the LAD2 cells to produce β-hexosaminidase.
[0059] The result is as image 3 Shown: PBS in the figure is the negative control group; CA8 / 80 is the positive control group. Wild-type Scy p 1 stimulated the release rate of β-hexosaminidase in LAD2 cells by 21%, while Scy p 1 derivatives could significantly reduce the ability of LAD2 cells to release β-hexosaminidase (LAD2 cells β-hexosam...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com