Antiviral andrographolide derivative and preparation method thereof
A technology of andrographolide and its derivatives, applied in antiviral agents, organic chemical methods, pharmaceutical formulations, etc., can solve the problems of low protein content, limited therapeutic effect of antiviral drugs, simple virus structure, etc., and achieve improved water solubility, The effect of improving bioavailability and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
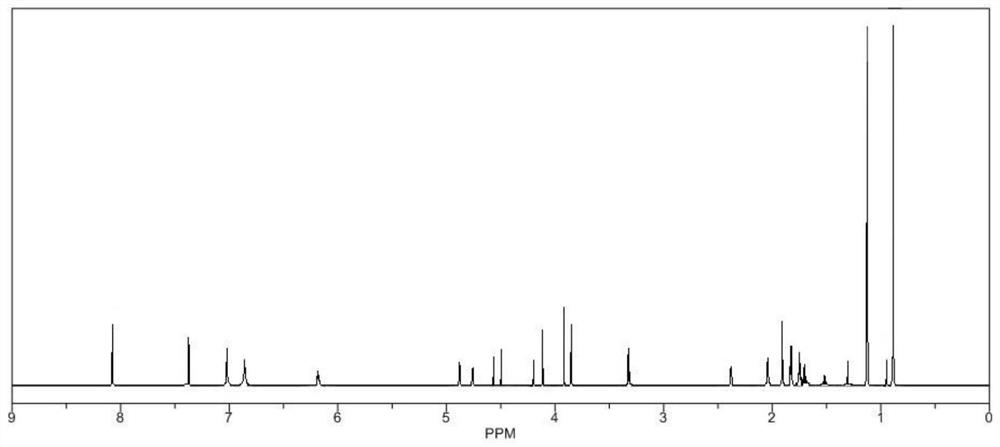
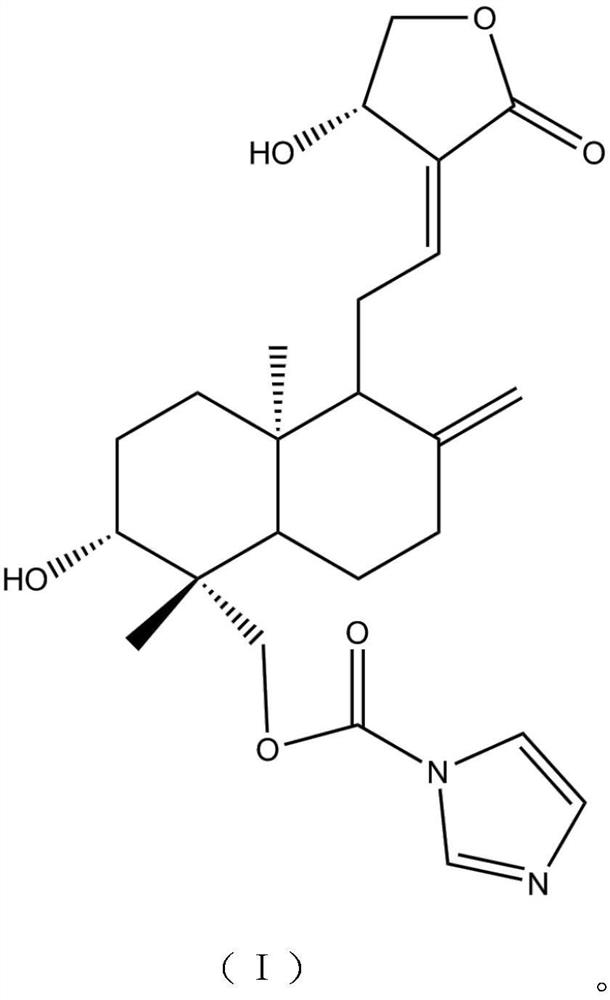
[0020] In a 100mL single-necked bottle, add 20g of andrographolide, add 40mL of tetrahydrofuran under the protection of nitrogen, stir to dissolve, adjust the temperature to 5°C, add 6g of N,N'-carbonyldiimidazole in batches, reflux for 4~6h, TLC Detect that the reaction is complete, stop the reaction, concentrate under reduced pressure at 60°C to recover the solvent, then add absolute ethanol, stir to dissolve, filter, and the filtrate is subjected to silica gel under the eluent ratio of petroleum ether:ethyl acetate=2:1 by volume. Column chromatography, the obtained chromatographic solution was concentrated, and then extracted with ethyl acetate, the ethyl acetate extract was concentrated, crystallized, filtered, and vacuum-dried to obtain the target product with a yield of 79.34%.
Embodiment 2
[0022] In a 100mL single-necked bottle, add 20g of andrographolide, add 40mL of tetrahydrofuran under nitrogen protection, stir to dissolve, adjust the temperature to 7°C, add 6g of N,N'-carbonyldiimidazole in batches, reflux for 4~6h, TLC Detect that the reaction is complete, stop the reaction, concentrate under reduced pressure at 60°C to recover the solvent, then add absolute ethanol, stir to dissolve, filter, and the filtrate is subjected to silica gel under the eluent ratio of petroleum ether:ethyl acetate=2:1 by volume. Column chromatography, the obtained chromatographic liquid was concentrated, and then extracted with ethyl acetate, the ethyl acetate extract was concentrated, crystallized, filtered, and vacuum-dried to obtain the target product with a yield of 80.56%.
Embodiment 3
[0024] In a 100mL single-necked bottle, add 20g of andrographolide, add 40mL of tetrahydrofuran under the protection of nitrogen, stir to dissolve, adjust the temperature to 9°C, add 6g of N,N'-carbonyldiimidazole in batches, reflux for 4~6h, TLC Detect that the reaction is complete, stop the reaction, concentrate under reduced pressure at 60°C to recover the solvent, then add absolute ethanol, stir to dissolve, filter, and the filtrate is subjected to silica gel under the eluent ratio of petroleum ether:ethyl acetate=2:1 by volume. Column chromatography, the obtained chromatographic solution was concentrated, and then extracted with ethyl acetate, the ethyl acetate extract was concentrated, crystallized, filtered, and vacuum-dried to obtain the target product with a yield of 80.77%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com