Oligonucleotide compositions and methods of use thereof
A technology of oligonucleotides and compositions, applied in biochemical equipment and methods, chemical instruments and methods, sugar derivatives, etc., can solve the problems of nucleic acid cell penetration and poor distribution, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
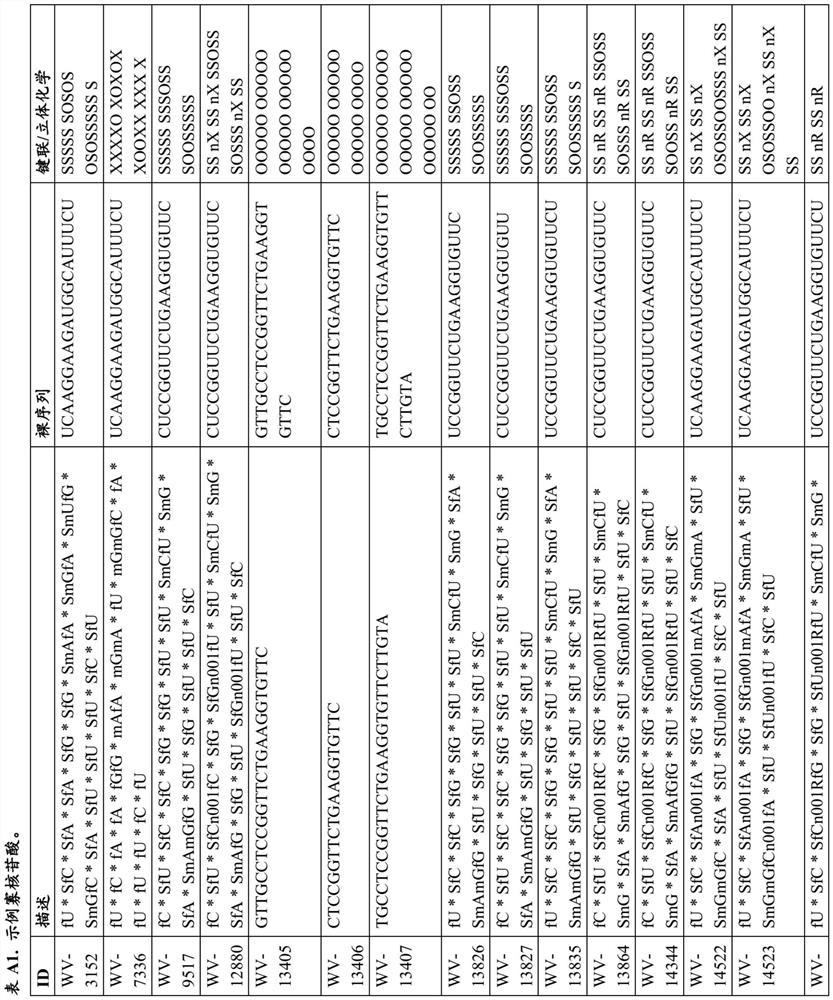
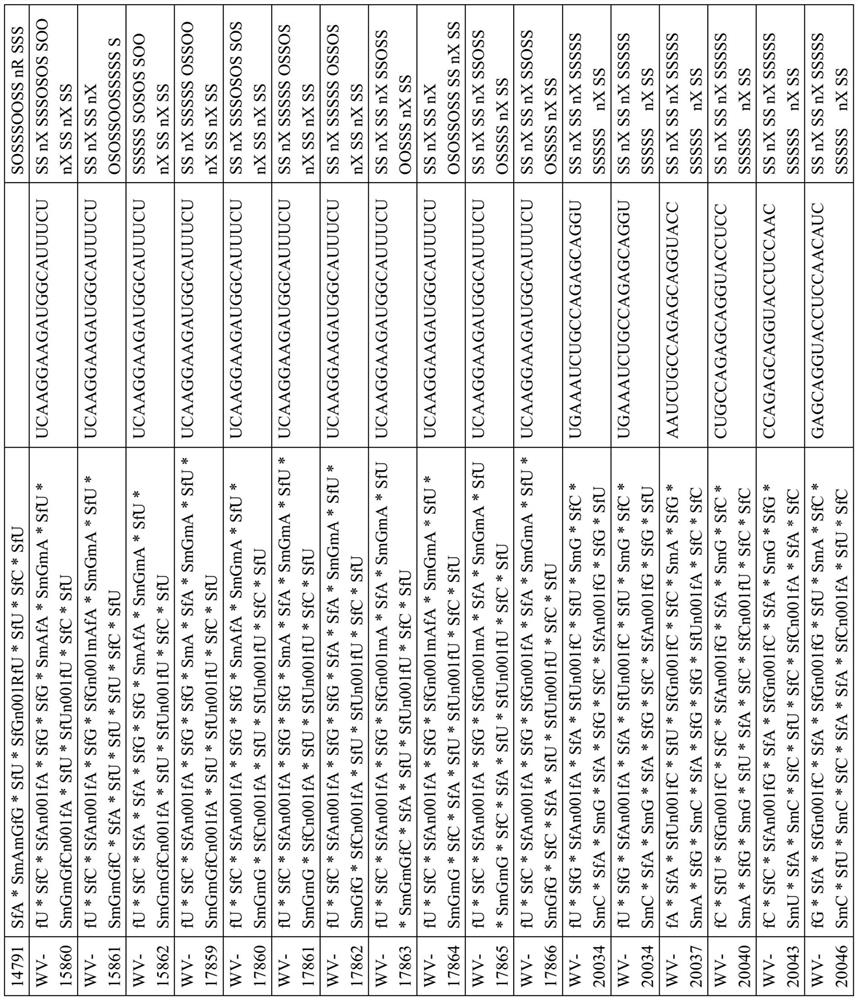
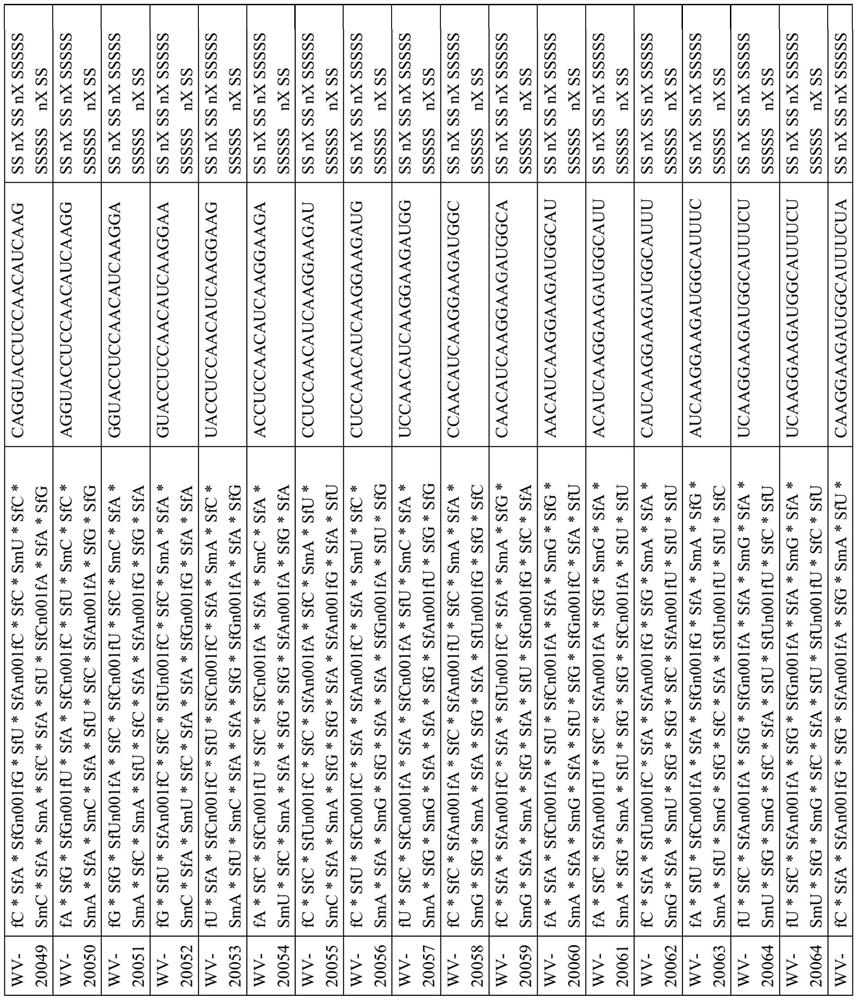
[1042] Example 1. Example Synthesis of DMD Oligonucleotide Compositions
[1043] Certain techniques for preparing DMD oligonucleotides and compositions thereof are widely known in the art. In some embodiments, techniques described in one or more of the following (e.g., reagents (e.g., solid supports, coupling reagents, cleavage reagents, phosphoramidites, etc.), chiral auxiliaries, solvents (e.g., , for reaction, washing, etc.), circulation, reaction conditions (for example, time, temperature, etc.) etc.) to prepare the DMD oligonucleotide and DMD oligonucleotide composition of the present disclosure: US 9394333, US9744183, US 9605019, US 9598458, US 2015 / 0211006, US 2017 / 0037399, WO 2017 / 015555, WO 2017 / 192664, WO 2017 / 015575, WO2017 / 062862, WO 2017 / 160741, PC US / TUS / 1961479, 8WO20 35687, PCT / US18 / 38835, and PCT / US18 / 51398.
example 2
[1044] Example 2. Exemplary synthesis of phosphoramidate internucleotide linkages comprising a cyclic guanidine moiety
[1045] As illustrated herein, according to the present disclosure, phosphoramidate internucleotide linkages can be readily prepared from phosphite internucleotide linkages, including stereopure phosphite internucleotide linkages.
[1046]
[1047] Amidite (474 mg, 0.624 mmol, 1.5 equiv, predried by co-evaporation with dry acetonitrile and under vacuum for a minimum of 12 h) and TBS-protected alcohol (150 mg, 0.41 mmol, via Co-evaporated with dry acetonitrile and pre-dried under vacuum for a minimum of 12h) To a stirred solution in dry acetonitrile (5.2ml) was added 5-(ethylthio)-1H-tetrazole (ETT, 2.08ml, 0.6M , 3 equivalents). The reaction mixture was stirred for 5 min and then monitored by LCMS before a solution of 2-azido-1,3-dimethylimidazoline hexafluorophosphate (356 mg, 1.24 mmol, 3 eq) in acetonitrile (1 ml) was added. Once the reaction was ...
example 31
[1054] in instance 31 In P NMR (phosphoric acid internal standard at δ0.0), the stereorandom preparation shows two peaks at -1.34 and -1.98, respectively; the stereopure Rp preparation shows a peak at -1.93, and the stereopure Sp preparation shows a peak at -1.38 peak.
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com