Fluorescent compound, and fluorescently-labeled biological substance using same
A compound and fluorescent technology, used in the field of fluorescent compounds and fluorescently labeled biological substances, can solve the problems of low light resistance, deterioration, and insufficient biological observation of organic fluorescent pigments
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0435] Hereinafter, the present invention will be described in further detail based on examples, but the present invention is not limited thereto.
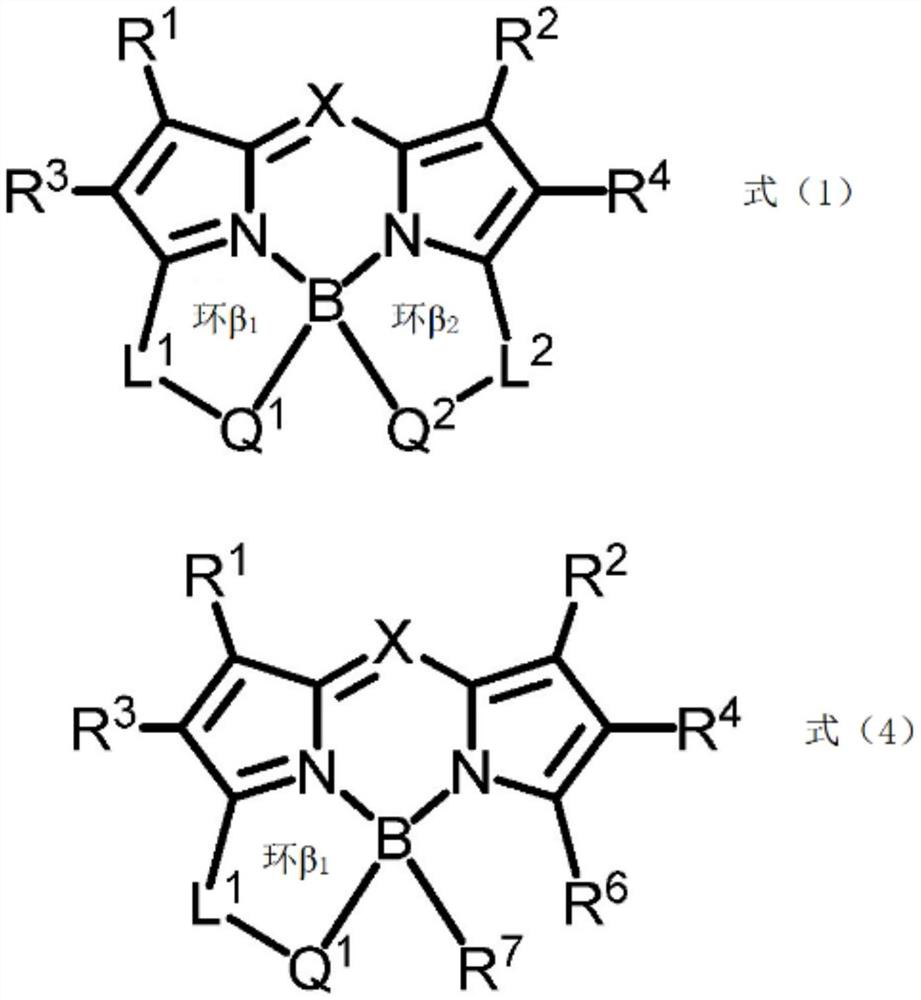
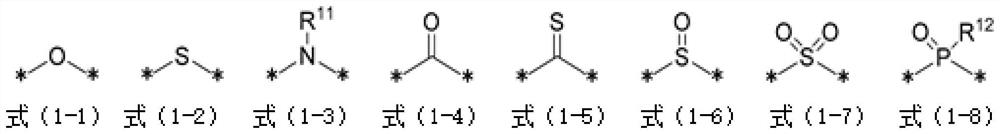
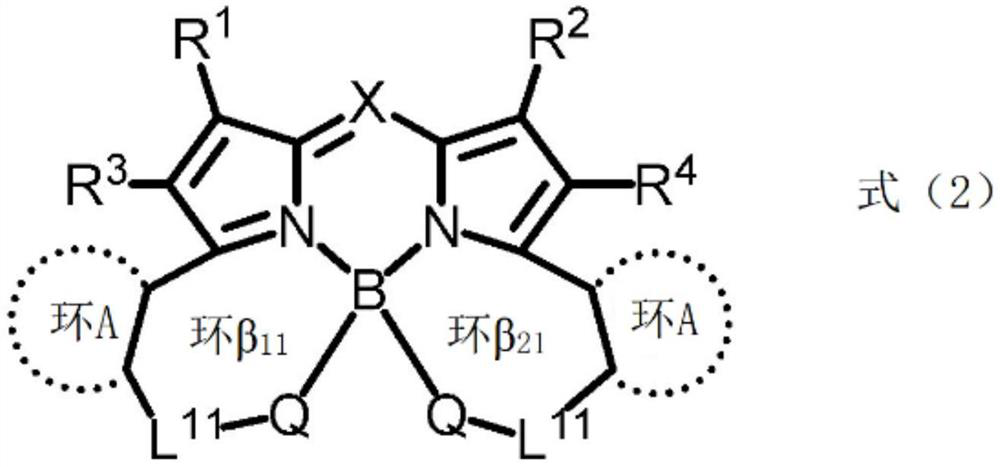
[0436] Compounds (1) to (24) and comparative compounds (1) to (2) used in Examples and Comparative Examples are shown below. In addition, [] in the structural formula of compound (14) represents a repeating structure.
[0437] [chemical formula 47]
[0438]
[0439] [chemical formula 48]
[0440]
[0441] Comparative compound (1) is compound A described in paragraph [0052] of International Publication No. 2013 / 035303.
[0442] The comparative compound (2) is BODIPY (registered trademark) 496 / 512 (manufactured by Invitrogen Japan K.K., product name).
[0443] Hereinafter, the synthesis methods of compounds (1) to (24) and labeled antibodies (1) to (12) used in each example will be described in detail, but the starting materials, dye intermediates, and synthesis routes are not limited to These.
[0444] Unless otherwise s...
Synthetic example 1
[0491] Compound (1) was synthesized based on the following scheme.
[0492] 1) Synthesis of compound (1-C)
[0493] The following compound (1-A), compound (1-B) and compound (1-C) were respectively according to the method described in Chem.Eur.J.2016,22,93-96, Org.Process Res.Dev. It was synthesized by the method described in 2015, 19, 1774-1783.
[0494] [chemical formula 49]
[0495]
[0496] 2) Synthesis of compound (1)
[0497] [chemical formula 50]
[0498]
[0499] 2-1) Synthesis of compound (1-D)
[0500] To 11 ml of a CPME solution of 112 mg of compound (1-C), 0.02 ml of water, 76 mg of 2-(hydroxymethyl)phenylboronic acid [Wako Pure Chemical Industries, Ltd.], PdCl 2 (dtbpf) 16 mg [Tokyo Chemical Industry Co., Ltd.] and cesium fluoride 152 mg [Wako Pure Chemical Industries, Ltd.] were stirred under reflux for 90 minutes. Next, after cooling the reaction liquid to room temperature, saturated sodium bicarbonate water was added to the reaction liquid, extracti...
Synthetic example 2
[0508] Compound (2) was synthesized based on the following scheme.
[0509] [chemical formula 51]
[0510]
[0511] In the synthesis method of compound (1), 2-(hydroxymethyl)phenylboronic acid was changed to 4-fluoro-2-(hydroxymethyl)phenylboronic acid [Combi-Blocks Inc.], and Compound (2) 1 mg (reddish-brown solid) was obtained in the same manner as the synthesis method of compound (1).
[0512] MS (ESI m / z): 557 (M+H)
[0513] RT(min): 1.28
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com