5-Substituent-1,2,4-triazole-thione Schiff base compound and preparation method and application thereof
A technology of substituents and Schiff bases, which is applied in the field of chemical drugs, can solve the problems of drug side effects, increased drug resistance, increased drug toxicity, and high toxicity, and achieve broad-spectrum high-efficiency bactericidal activity, reduced drug concentration, and high toxicity. Effect with few side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~3
[0052] Examples 1-3 use intermediate (I) to synthesize 5-substituent-1,2,4-triazole-thione Schiff base compound
Embodiment 1
[0053] Preparation and characterization of embodiment 1 compound TM1
[0054] Synthesis of 4-(3,5-Dimethyl-4-hydroxybenzylideneamino)-5-methyl-2H-1,2,4-triazole-3(4H) by Intermediate (Ⅰ-1) - Thione (Compound TM1). The preparation method comprises the following steps:
[0055] (1) Synthesis of 4-amino-5-methyl-2H-1,2,4-triazole-3(4H)-thione intermediate (I-1)
[0056] Complete through the following synthetic routes, specifically:
[0057]
[0058] (2) Synthesis of 5-methyl-2H-1,2,4-triazole-3(4H)-thione Schiff base compound TM1
[0059] Complete through the following synthetic routes, specifically:
[0060]
[0061] In summary, the specific steps of using intermediate (I-1) to synthesize compound TM1 are as follows:
[0062] Weigh 141 mmol of thiocarbazide, add excess acetic acid to carry out nucleophilic substitution reaction, filter the solution obtained from the reaction, wash, and recrystallize to obtain intermediate (I-1);
[0063] The above-mentioned intermedi...
Embodiment 2
[0068] Preparation and characterization of embodiment 2 compound TM2
[0069] Compound TM2 was synthesized using intermediate (I-2).
[0070] The preparation method of compound TM2 can refer to the preparation method of TM1 in Example 1. But different from Example 1, the fatty acid used is propionic acid, and the yield of compound TM2 is 71.2%.
[0071] Further, the properties of compound TM2 were characterized.
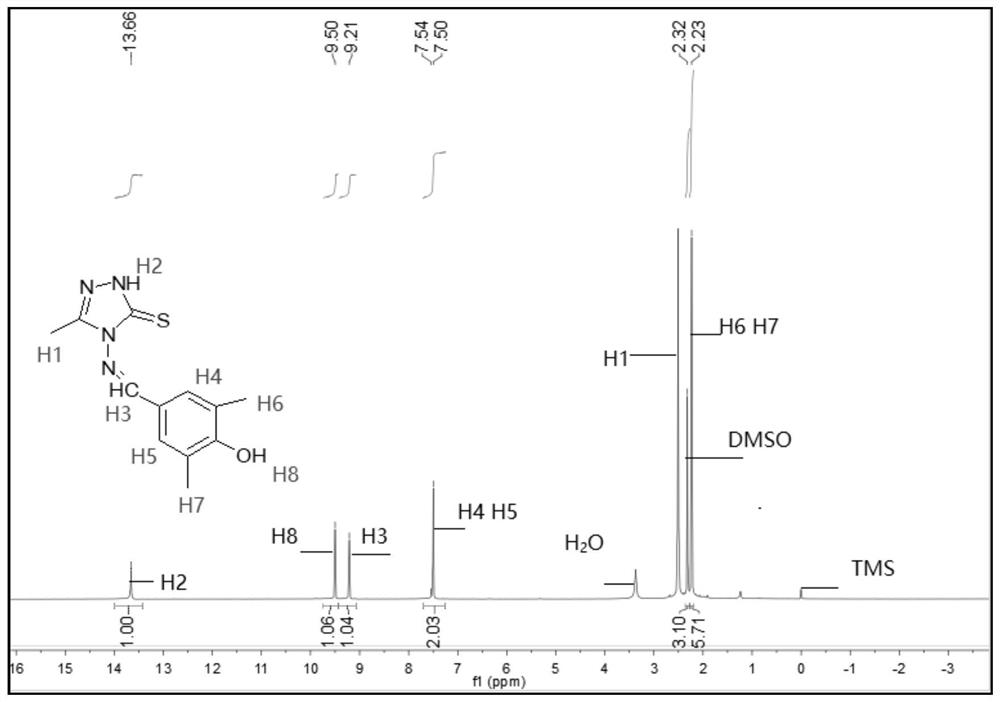
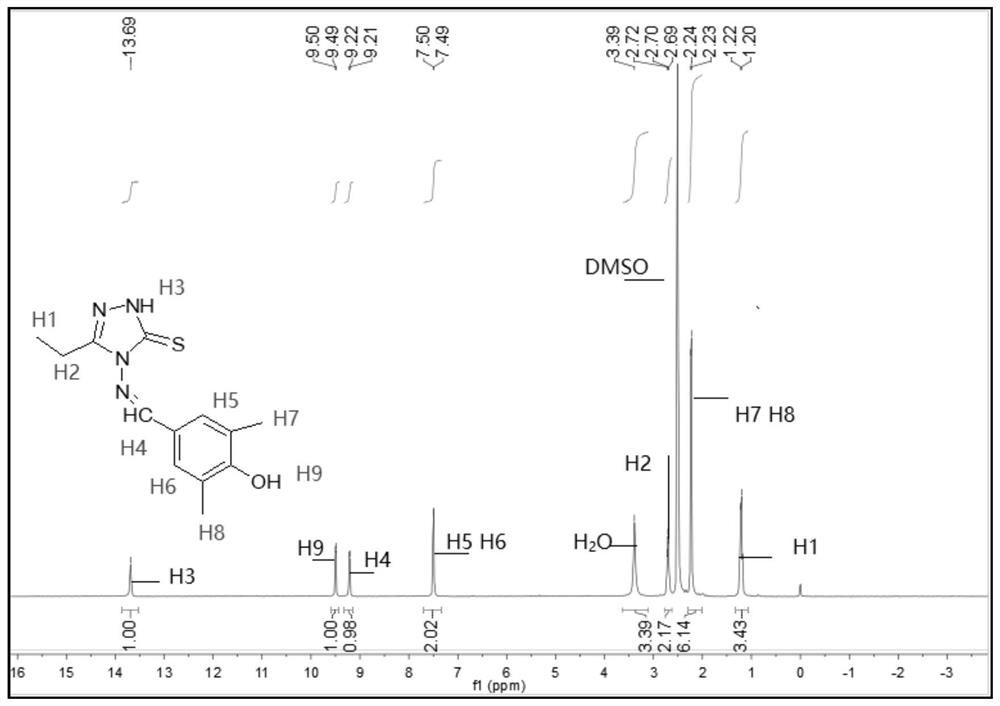
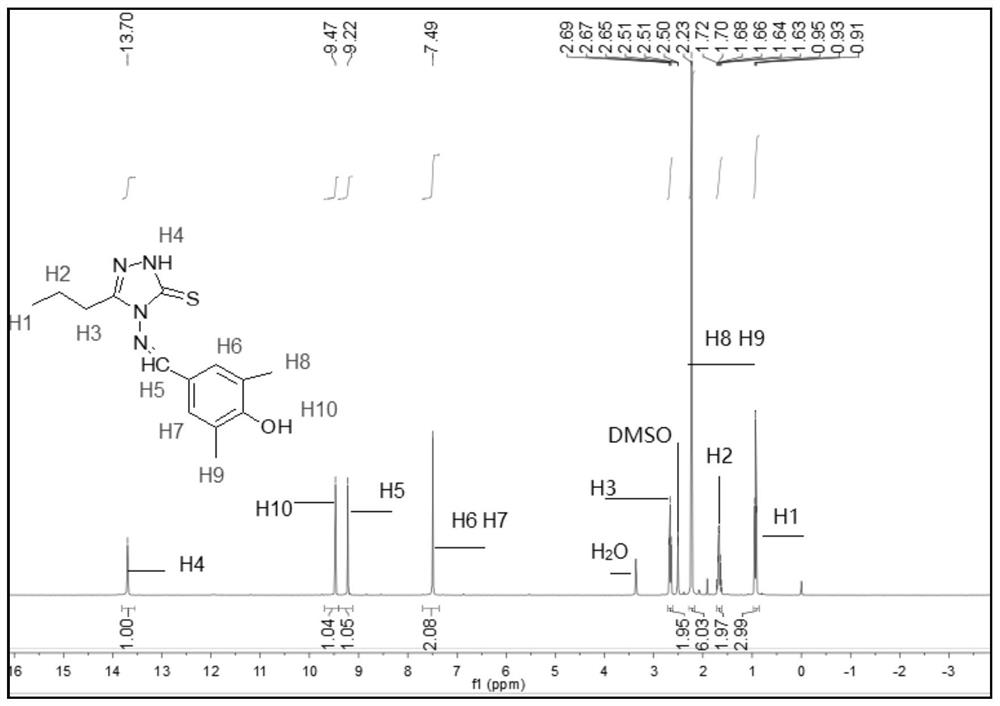
[0072] The melting point of compound TM2 is determined to be 218.1-219.6°C, and it is detected by nuclear magnetic resonance to obtain 1 H NMR spectrum, such as figure 2 shown.
[0073] see figure 2 , 1 H NMR (400MHz, DMSO-d 6 ,ppm)δ13.69(s,1H,NH),9.49(d,J=5.4Hz,1H,OH),9.21(d,J=5.1Hz,1H,CH=N),7.49(d,J= 4.8Hz,2H,benzene),3.39(s,3H,CH 3 ),2.98-2.56(m,2H,CH 2 ), 2.23 (d, J=5.1Hz, 6H, CH 3 );IR(KBr,cm -1 ): ν: 3408(N-H), 3062(OH), 1574(C=N); Anal.Calcd(%) for C 13 h 16 N 4 OS (276.36): C, 56.50; H, 5.84; N, 20.27. Found: C, 56.43; H, 5.45; N, 20.20.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com