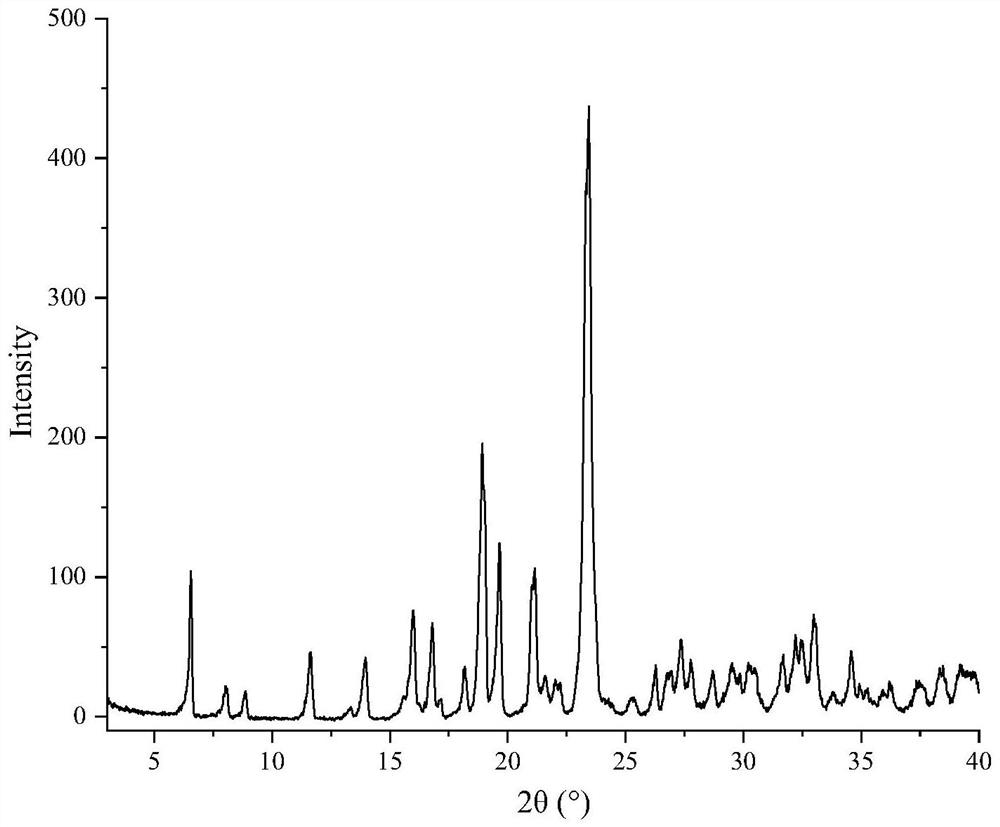
Puerarin anhydrous spherical crystal and preparation method and application thereof
A technology of spherical crystals and puerarin, applied in the field of medicine, can solve problems such as unfavorable development of solid preparations, achieve good development prospects, simple operation, and improve fluidity and compressibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Preparation of Spherical Crystals of Puerarin Anhydrate
[0050] Add 150 mL of acetonitrile to the crystallization container, place it in a constant temperature water bath to keep the temperature at 30°C, turn on the stirrer and keep the speed at 250 rpm; weigh about 1 g of puerarin, put it in a vial, add 10 mL of methanol, and ultrasonically dissolve the drug Dissolved, the resulting solution was added dropwise to the crystallization container, and the timer was started after the dropwise addition was completed, and the stirring was kept for 60 min. Suction filtration was performed on the suspension, and the solid was vacuum-dried at 25°C for 24 h, and the obtained dried solid particles were the product.
[0051] The appearance of the product is spherical, the Carr index is 10.92%, the angle of repose is 24.7°, and the average particle size is 75 μm.
Embodiment 2
[0053] Preparation of Spherical Crystals of Puerarin Anhydrate
[0054] Add 50 mL of acetonitrile to the crystallization vessel, place it in a constant temperature water bath to keep the temperature at 35 °C, turn on the stirrer and keep the speed at 500 rpm; weigh about 0.2 g of puerarin, put it in a vial, add 10 mL of methanol, and ultrasonically dissolve the drug Dissolved, the resulting solution was added dropwise to the crystallization container, and the timer was started after the dropwise addition was completed, and the stirring was kept for 90 min. The suspension was suction filtered, and the solid was vacuum-dried at 30°C for 12 h, and the obtained dried solid particles were the product.
[0055] The appearance of the product is spherical, the Carr index is 15.43%, the angle of repose is 28.7°, and the average particle size is 43 μm.
Embodiment 3
[0057] Preparation of Spherical Crystals of Puerarin Anhydrate
[0058] Add 150 mL of acetonitrile to the crystallization vessel, place it in a constant temperature water bath to keep the temperature at 20°C, turn on the stirrer and keep the rotation speed at 100 rpm; weigh about 1 g of puerarin, put it in a vial, add 5 mL of methanol, and ultrasonically dissolve the drug Dissolved, the resulting solution was added dropwise to the crystallization vessel, and the timer was started after the dropwise addition was completed, and the stirring was kept for 10 min. The suspension was suction filtered, and the solid was vacuum-dried at 20°C for 48 h, and the obtained dried solid particles were the product.
[0059] The appearance of the product is spherical, the Carr index is 18.48%, the angle of repose is 29.5°, and the average particle size is 84 μm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Angle of repose | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| Angle of repose | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com