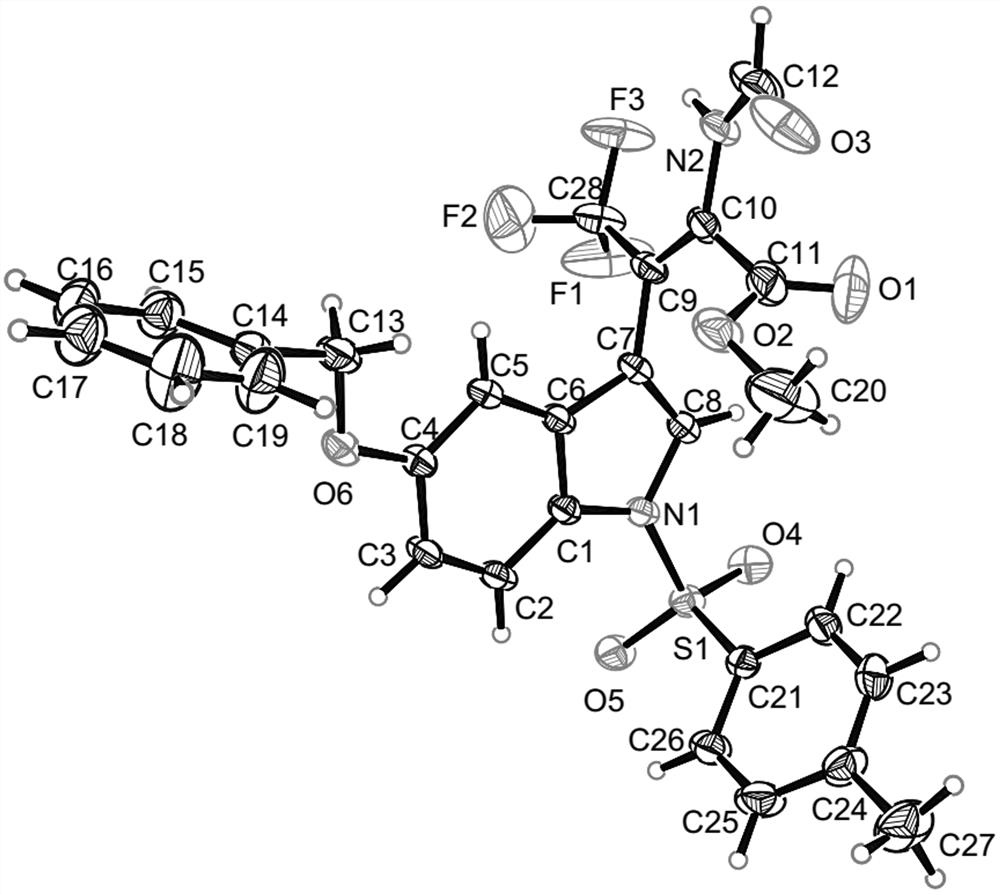
(Z)-beta-trifluoromethyl dehydrotryptophan compound and synthesis method and application thereof
A technology of trifluoromethyl group and synthesis method, applied in the field of organic synthesis, can solve the problems of poor atom economy, high operation requirements, low yield and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
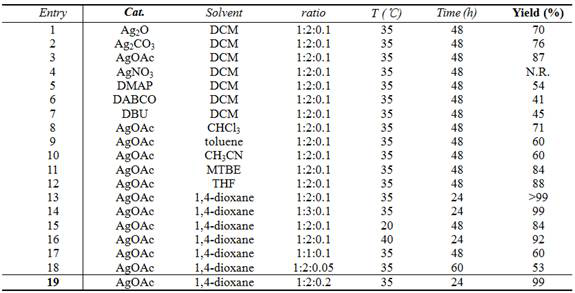
[0030] Catalyst screening experiments: Different catalysts promote the reaction of 3-trifluoroacetyl indole with isocyanate, resulting in different yields.
[0031] Pick N - p-toluenesulfonyl-3-trifluoroacetylindole (0.2 mmol), methyl isocyanate (0.4 mmol), catalyst (0.02 mmol), added to 2 mL of dichloromethane, 35 ° C for 48 hours, the reaction After completion, the solvent was removed under reduced pressure, and compound A was obtained by column chromatography.
[0032]
[0033]
[0034] The ratio in the table refers to the N - p-toluenesulfonyl-3-trifluoroacetylindole:methyl isocyanate:catalyst ratio.
Embodiment 2
[0036] The preparation method of compound A with the following structure:
[0037]
[0038] Pick N - p-toluenesulfonyl-3-trifluoroacetylindole (0.2 mmol), methyl isocyanate (0.4 mmol), AgOAc (0.02 mmol), added to 2 mL 1,4-dioxane, 35 °C The reaction was carried out for 24 hours. After the reaction was completed, the solvent was removed under reduced pressure, and 92.3 mg of compound A was obtained as a white solid through column chromatography separation, with a yield of >99%.
[0039] NMR characterization is as follows: 1 H NMR (400 MHz, CDCl 3 ) δ 8.27 (s, 1H), 8.14 (s, 1H),7.97 (d, J= 8.3 Hz, 1H), 7.77 (d, J = 8.2 Hz, 2H), 7.58 (s, 1H), 7.44 (d, J = 7.8 Hz, 1H), 7.34 (d, J = 7.8 Hz, 1H), 7.26 (t, J = 7.1 Hz, 3H), 3.19 (s,3H), 2.34 (s, 3H); 13 C NMR (100 MHz, CDCl 3 ) δ 162.9, 158.0, 145.4, 134.9, 134.4, 134.3, 130.0, 130.0, 127.5, 126.9, 125.4, 123.9, 123.2 (q, J = 275.1Hz, 1C), 120.4, 113.5, 112.2, 108.9 (q, J = 32.6 Hz, 1C), 52.4, 21.6.
Embodiment 3
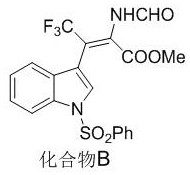
[0041] The preparation method of compound B with the following structure:
[0042]
[0043] Pick N -Phenylsulfonyl-3-trifluoroacetylindole (0.2 mmol), methyl isocyanate (0.4 mmol), AgOAc (0.02 mmol), add 2 mL 1,4-dioxane, and react at 35°C After 24 hours, after the reaction was completed, the solvent was removed under reduced pressure, and 87.7 mg of compound B was obtained by column chromatography separation as a colorless oil, with a yield of 97%.
[0044] NMR characterization is as follows: 1 H NMR (400 MHz, CDCl 3 ) δ 8.20 (s, 1H), 7.96 (s, 1H), 7.92 (d, J = 8.3 Hz, 1H), 7.85-7.78 (m, 2H), 7.50 (s, 1H), 7.48 (d, J = 7.4Hz, 1H), 7.39 (dd, J = 16.9, J = 8.4 Hz, 3H), 7.28 (d, J = 7.8 Hz, 1H), 7.24-7.19 (m, 1H), 3.07 (s, 3H); 13 C NMR (100 MHz, CDCl 3 ) δ162.8, 157.8, 137.9,134.5, 134.3, 134.2, 130.0, 129.5, 127.5, 126.9, 125.5, 124.0, 123.2 (q, J =275.1 Hz, 1C), 120.5, 113.5, 112.4, 108.7 (q, J = 33.5 Hz, 1C), 52.5.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com