Method for efficiently expressing AFP3-PTEN fusion protein
A technology of AFP3-PTEN and fusion protein, applied in the direction of pregnancy protein, mammalian protein, chemical instruments and methods, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
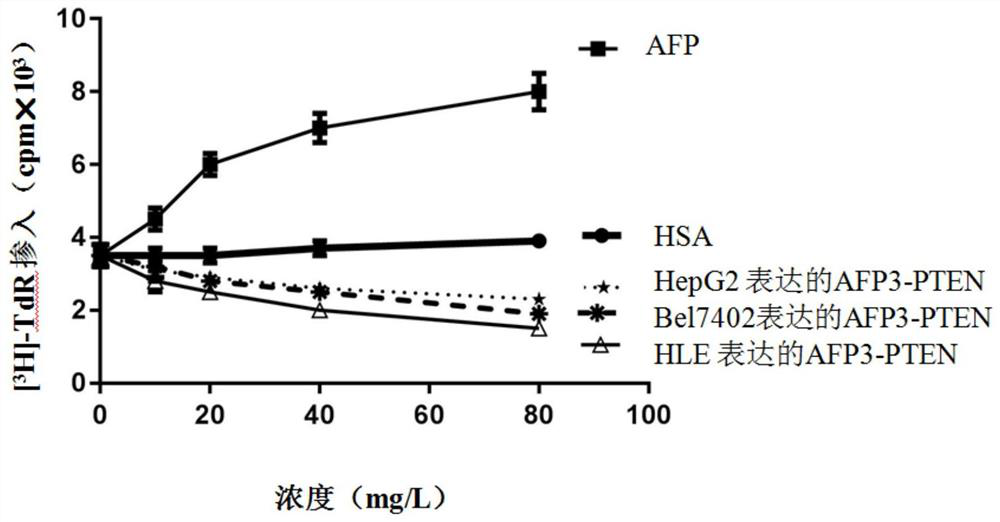
[0026] Embodiment 1 Utilizes the method of high-efficiency expression and purification of AFP3-PTEN fusion protein by HLE cells
[0027] 1. Establishment of HLE cells expressing HBx
[0028] According to the human HBx gene sequence (NCBI accession number is AB210819), the synthetic gene was double-digested with Xba I and Xho I, connected to the expression vector PTT5 (Invitrogen) with T4 ligase, transformed into DH5α cells, extracted the plasmid, and then transfected into HLE Cells were cultured for 48 hours to detect the expression of HBx protein.
[0029] 2. The plasmid containing PTT5-AFP3-PTEN was transfected into HLE cells expressing HBx, cultured for 48 hours, and the culture medium was collected.
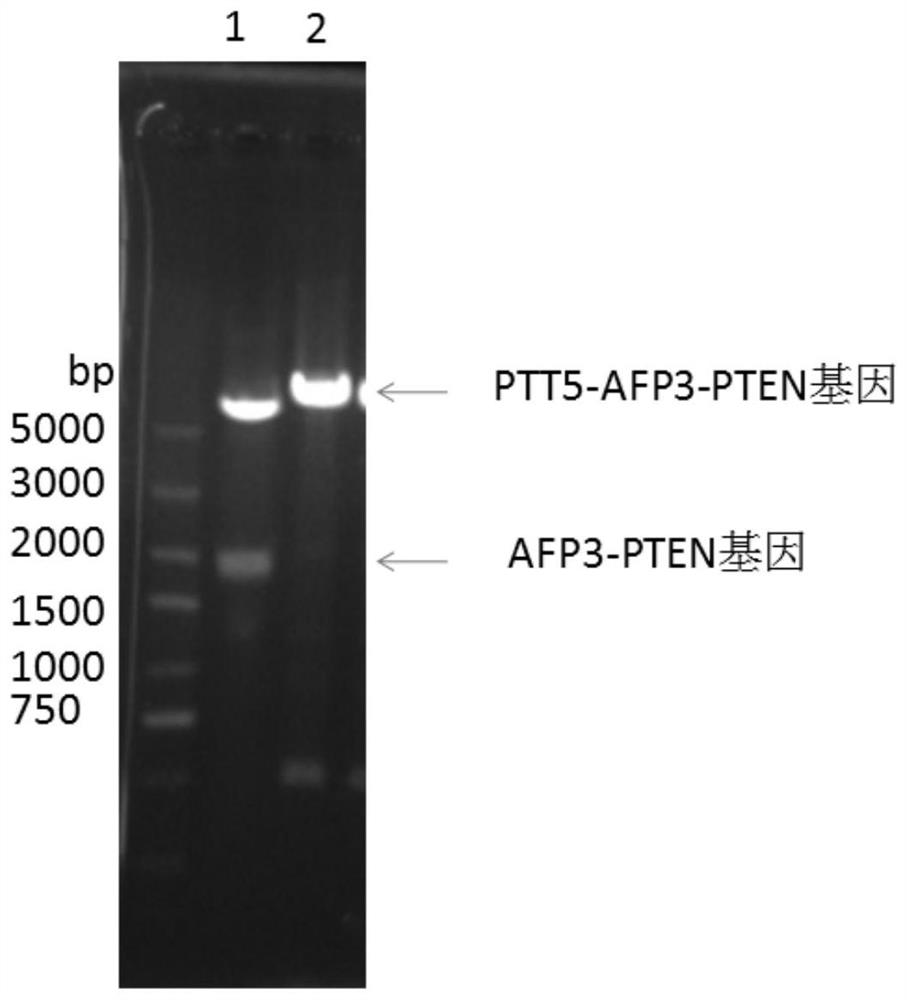
[0030] 3. Separation, purification and identification of AFP3-PTEN fusion protein. Since the protein is secreted, the medium and cell mixture was centrifuged at 8000r / min for 5min, and the supernatant was concentrated and switched through a tangential flow membrane. The sol...
Embodiment 2
[0033] Embodiment 2 Utilizing HepG2 cells to efficiently express and purify the method of AFP3-PTEN fusion protein
[0034] 1. Establishment of HepG2 cells expressing HBx
[0035] According to the human HBx gene sequence (NCBI accession number is AB210819), the synthetic gene was double-digested with Xba I and Xho I, connected to the expression vector PTT5 (Invitrogen) with T4 ligase, transformed into DH5α cells, extracted the plasmid, and then transfected into HepG2 Cells were cultured for 48 hours to detect the expression of HBx protein.
[0036] 2. The plasmid containing PTT5-AFP3-PTEN was transfected into HepG2 cells expressing HBx, cultured for 96 hours, and the medium was collected.
[0037] 3. Separation, purification and identification of recombinant proteins. After the medium and cell mixture was centrifuged at 8000r / min for 5min, the supernatant was taken to concentrate the switching solution through a tangential flow membrane, and the solution was HBS buffer (10mM...
Embodiment 3
[0040] Embodiment 3 Utilizing Bel-7402 cells to efficiently express and purify the method of AFP3-PTEN fusion protein
[0041] 1. Establishment of Bel-7402 cells expressing HBx
[0042] According to the human HBx gene sequence (NCBI accession number is AB210819), the synthetic gene was digested with Xba I and Xho I, connected to the expression vector PTT5 (Invitrogen) with T4 ligase, transformed into DH5α cells, extracted the plasmid, and then transfected into Bel -7402 cells were cultured for 72 hours to detect the expression of HBx protein.
[0043] 2. The plasmid containing PTT5-AFP3-PTEN was transfected into Bel-7402 cells expressing HBx, cultured for 85 hours, and the medium was collected.
[0044] 3. Separation, purification and identification of recombinant proteins. Since the protein is secreted and expressed, the culture medium and the cell mixture were centrifuged at 8000r / min for 5min, and the supernatant was lyophilized and replaced with HBS (10mM Hepes, pH 7.2, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com