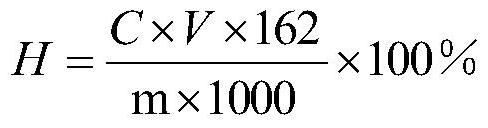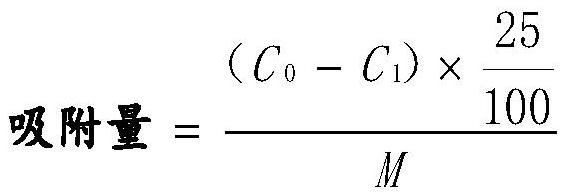Dialdehyde cellulose with high aldehyde group content as well as preparation method and application thereof
A technology of dialdehyde cellulose and cellulose, which is applied in the field of functional polymers and natural polymers, can solve the problems of low accessibility, poor reaction uniformity, decreased product yield, etc., and achieves improved yield drop, uniform product, Simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
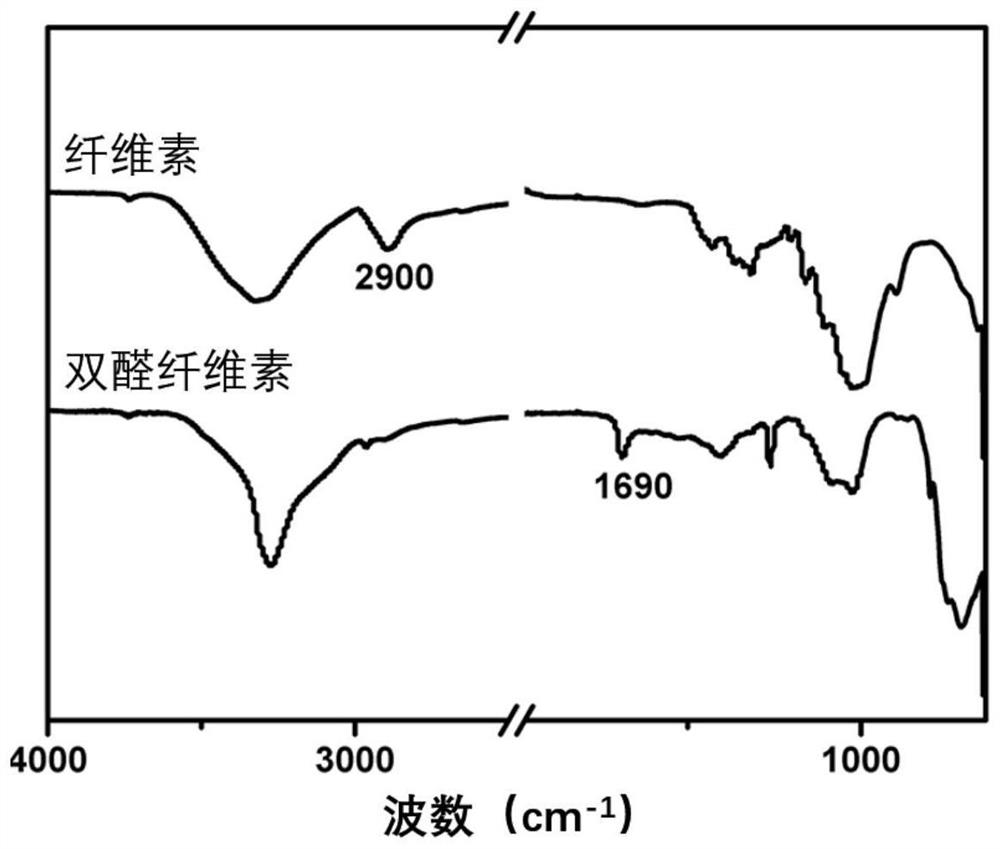
[0043] Weigh 4g of dry microcrystalline cellulose, whose degree of polymerization is about 220, add it to 36g of ionic liquid 1-allyl-3-methylimidazolium chloride (AmimCl), stir and dissolve at 80°C to obtain a mass fraction of 10% Cellulose clear clear solution. The reaction flask was tightly covered with tin foil to avoid light, and then 10.56 g of sodium periodate was added, and stirred and reacted at 80° C. for 8 hours. After the reaction, ethanol was added to precipitate, and the crude product obtained was washed three times with ethylene glycol and then dried to obtain the product dialdehyde cellulose powder. The product was tested by infrared with a Perkin-Elmer2000 infrared spectrometer with a resolution of 4 cm. -1 , the infrared spectrum as figure 1As shown, of which 1629cm -1 A new peak appeared, proving the formation of aldehyde group.
[0044] The aldehyde group content of the product is determined by the hydroxylamine hydrochloride method: add 100mL of hydroxy...
Embodiment 2
[0054] Take by weighing 3g dry absorbent cotton, its degree of polymerization is about 1300, join in the mixed solvent of 57g ionic liquid 1-ethyl-3-methylimidazole acetate (EmimAc) and DMSO (the massfraction of DMSO in the mixed solvent is 10 %), stirred and dissolved at 80°C to obtain a transparent and clear solution of cellulose with a mass fraction of 5%. Tightly cover the reaction flask with tin foil to avoid light, add 7.92g of sodium periodate, stir and react at 60°C for 8 hours to obtain a solution, add deionized water to the reaction solution for precipitation washing, filter to obtain the crude product, and use ethylene glycol Stirring and washing for three times, drying to obtain the product dialdehyde cellulose powder. The aldehyde content of the product and the determination method of the yield were measured according to the description in Example 1, and the aldehyde content was measured to be 82%, and the product yield was 98%.
[0055] Repeat the above reaction...
Embodiment 3
[0057] Take by weighing 3g dry cotton pulp, its degree of polymerization is about 800, join in the mixed solvent of 57g ionic liquid 1-butyl-3-methylimidazole acetate (BmimAc) and methylimidazole (methylimidazole in mixed solvent The mass fraction of imidazole is 15%), stirred at 60° C. to obtain a cellulose transparent and clear solution with a mass fraction of 5%. The reaction flask was tightly covered with tin foil to avoid light, and then 7.92 g of sodium periodate was added, and stirred and reacted at 80° C. for 10 hours. After the reaction is completed, add deionized water to precipitate and wash, filter to obtain the crude product, stir and wash with ethylene glycol for three times, and then dry to obtain the product dialdehyde cellulose powder. The aldehyde content of the product and the determination method of the yield were measured according to the description in Example 1, and the aldehyde content was measured to be 90%, and the product yield was 97%.
[0058] Dis...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Adsorption capacity | aaaaa | aaaaa |
| Adsorption capacity | aaaaa | aaaaa |
| Adsorption capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com