Internet-based real-time shared blood screening indoor quality control system and method
An indoor quality control and blood screening technology, which is applied in the field of blood screening indoor quality control system, can solve the problems of incomplete application and inability to purchase, and achieve the effect of easy use, rapid response, and unified quality control standards
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
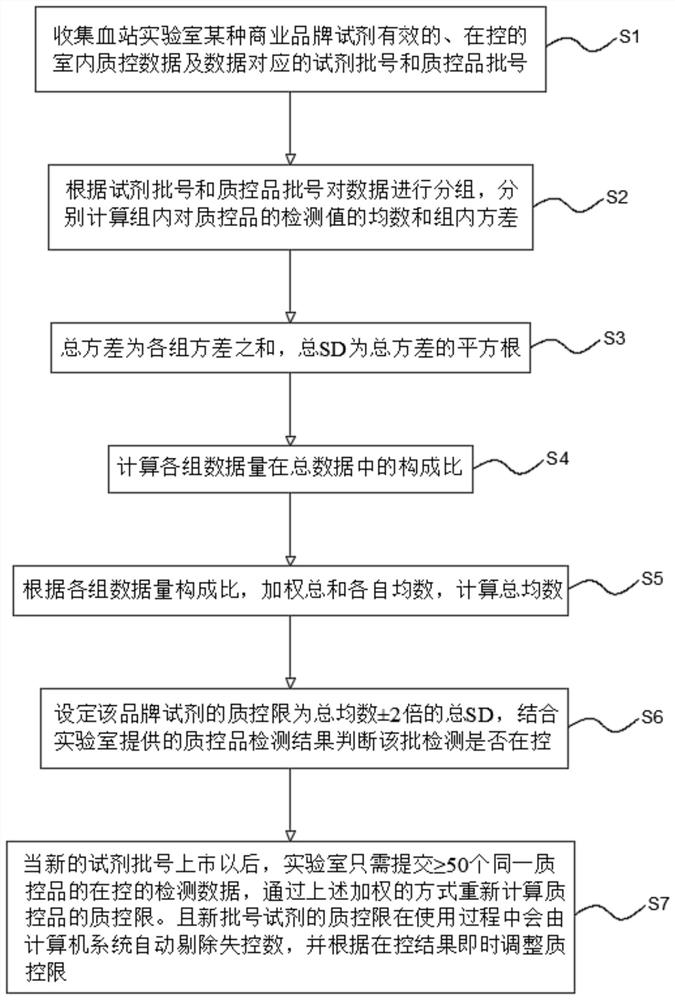
[0047] Embodiment 1 The present invention's Internet-based real-time shared blood screening indoor quality control method (1)
[0048] see figure 1 , the Internet-based real-time shared blood screening indoor quality control method of the present embodiment includes the following steps:
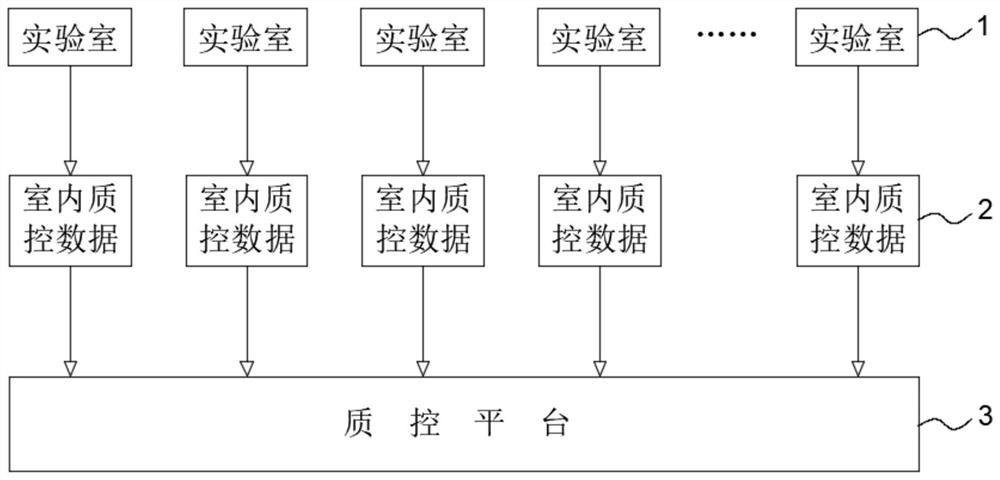
[0049] S1. Collect the effective and under-controlled indoor quality control data of a certain commercial brand reagent in the blood bank laboratory and the corresponding reagent batch number and quality control product batch number. The data volume of each commercial brand reagent must be ≥ 200. Participate in data collection The number of laboratories must be ≥ 3.
[0050] S2, the data are grouped according to the batch number of the reagent and the batch number of the quality control substance, and the mean and the variance within the group are calculated respectively. The amount of data in each group must be ≥50.
[0051] S3, the total variance is the sum of the variances of each group...
Embodiment 2
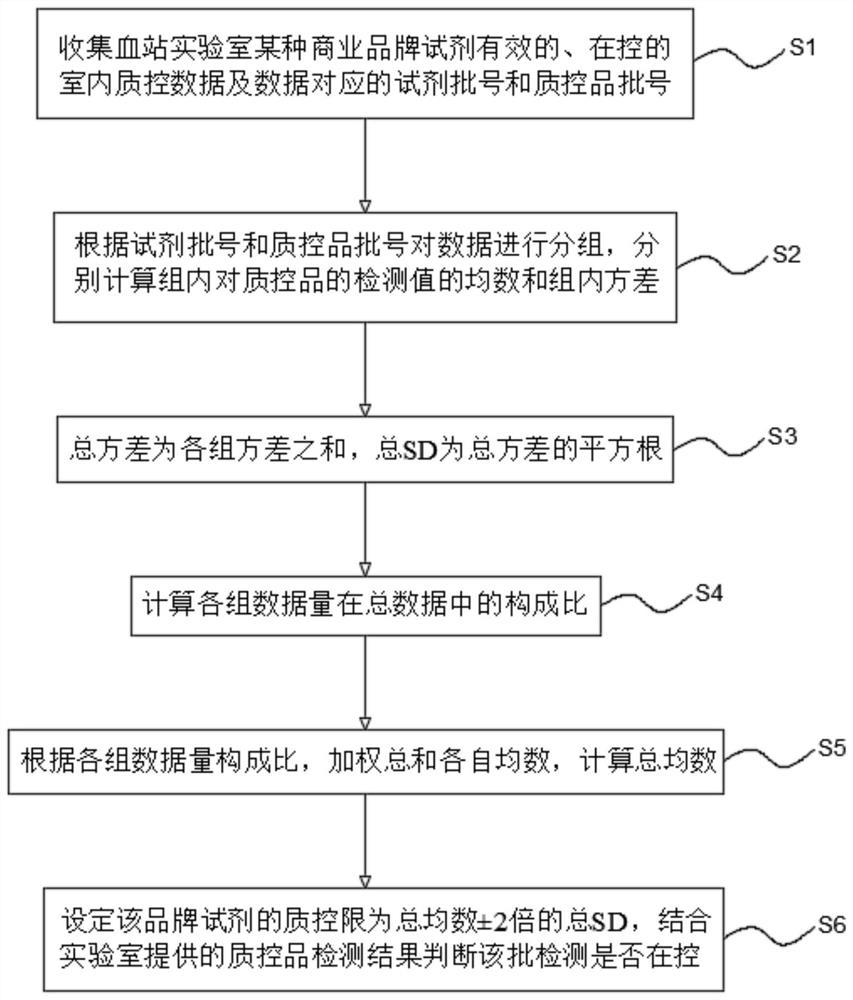
[0080] Embodiment 2 The present invention's Internet-based real-time shared blood screening indoor quality control method (2)
[0081] see figure 2 , the Internet-based real-time shared blood screening indoor quality control method of the present embodiment includes the following steps:
[0082] S1. Collect the effective and under-controlled indoor quality control data of a certain commercial brand reagent in the blood bank laboratory and the corresponding reagent batch number and quality control product batch number. The data volume of each commercial brand reagent must be ≥ 200. The number of laboratories participating in data collection for branded reagents must be ≥3.
[0083] S2, grouping the data according to the batch number of the reagent and the batch number of the quality control substance, and calculating the mean and variance within the group of the detection values of the quality control substance within the group. The amount of data in each group must be ≥50. ...
Embodiment 3
[0089] Embodiment 3 application example
[0090] From January to August 2019, 4 blood stations in Shanghai Blood Center, Shenzhen Blood Center, Shandong Blood Center and Dalian Blood Center used Roche COBAS s201 MPX v2.0 reagent to detect NRLQConnect quality control by mixed sample detection method Taking the HCV detection data of the product as an example, compare the difference between the patent of the present invention and the Australian quality control analysis software. The materials and methods are basically the same as the article "Discussion on the Application of Internet-Based Real-time Sharing Indoor Quality Control Technology in Blood Screening Nucleic Acid Detection" published by the applicant in "Chinese Journal of Blood Transfusion" Volume 32, Issue 9, 2019. The time is extended, and some laboratory data are more substantial than the original text.
[0091] S1. Collect the HCV detection data of the NRLQConnect quality control product in the Roche COBAS s201 MPX...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com