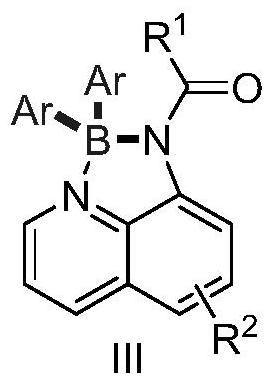
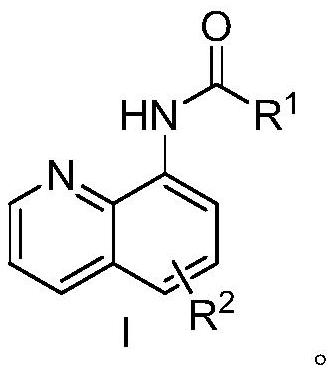
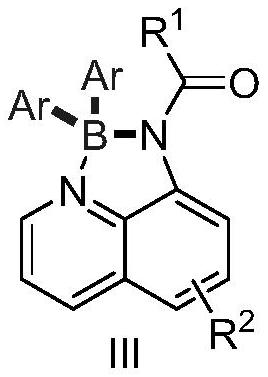
Preparation method of tetra-coordinated N,N-chelated diarylborate compound with 8-aminoquinoline derivatives as bidentate ligand
An aminoquinoline and bidentate ligand technology is applied in the field of preparation of four-coordinate N,N-chelated diarylboronic acid esters, which can solve the problems of harsh reaction conditions, poor functional group compatibility and low synthesis efficiency. , to achieve the effect of good raw material stability, high compatibility, and reduction of separation and purification steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] 1-(2,2-Diphenyl-2λ 4 ,3λ 4 Preparation of -[1,3,2]diazaborolin[4,5,1-ij]quinolin-1(2H)-yl)-3-phenylpropan-1-one
[0049]
[0050] This example prepares 1-(2,2-diphenyl-2λ at the milligram level 4 ,3λ 4 -[1,3,2]diazabororine[4,5,1-ij]quinolin-1(2H)-yl)-3-phenylpropan-1-one, under air conditions, the N-phenylpropionyl-8-aminoquinoline (41.4 mg, 0.15 mmol, 1.0 equiv) and potassium phenyltrifluoroborate (138.0 mg, 0.75 mmol, 5.0 equiv), manganese (24.7 mg, 0.45 mmol, 3.0 equiv) , 4-toluenesulfonyl chloride (71.5mg, 0.375mmol, 2.5equiv), sodium carbonate (7.9mg, 0.075mmol, 0.5equiv), acetonitrile (1.5mL) were added to a pressure-resistant reaction flask, and reacted at 130°C for 24 hours . After the reaction, the reaction mixture was filtered, washed with dichloromethane, and the solvent was removed by rotary evaporation, and then purified by silica gel column chromatography (the silica gel specification was 200 to 300 mesh, the mass ratio of silica gel to the produc...
Embodiment 2
[0054] 1-(2,2-Di-p-tolyl-2λ 4 ,3λ 4 Preparation of -[1,3,2]diazaborolin[4,5,1-ij]quinolin-1(2H)-yl)-3-phenylpropan-1-one
[0055] Under air conditions, N-phenylpropionyl-8-aminoquinoline (41.4mg, 0.15mmol, 1.0equiv) and potassium 4-tolyltrifluoroborate (148.5mg, 0.75mmol, 5.0equiv), manganese (24.7 mg, 0.45mmol, 3.0equiv), 4-toluenesulfonyl chloride (71.5mg, 0.375mmol, 2.5equiv), sodium carbonate (7.9mg, 0.075mmol, 0.5equiv), acetonitrile (1.5mL) were added in a pressure-resistant reaction flask , reacted at 130°C for 24 hours. After the reaction, the reaction mixture was filtered, washed with dichloromethane, and the solvent was removed by rotary evaporation, and then purified by silica gel column chromatography (the silica gel specification was 200 to 300 mesh, the mass ratio of silica gel to the product to be purified was 200:1, and the eluent was petroleum ether and ethyl acetate volume ratio is 3:1 mixed liquor), obtain target product 1-(2,2-two-p-tolyl-2λ 4 ,3λ 4 -[...
Embodiment 3
[0058] 1-(2,2-bis(4-methoxyphenyl)-2l 4 ,3l 4 Preparation of -[1,3,2]diazaborolin[4,5,1-ij]quinolin-1(2H)-yl)-3-phenylpropan-1-one
[0059] Under air conditions, N-phenylpropionyl-8-aminoquinoline (41.4 mg, 0.15 mmol, 1.0 equiv) and potassium 4-methoxyphenyl trifluoroborate (160.5 mg, 0.75 mmol, 5.0 equiv), Manganese (24.7mg, 0.45mmol, 3.0equiv), 4-toluenesulfonyl chloride (71.5mg, 0.375mmol, 2.5equiv), sodium carbonate (7.9mg, 0.075mmol, 0.5equiv), acetonitrile (1.5mL) were added under pressure In the reaction flask, the reaction was carried out at 130° C. for 24 hours. After the reaction, the reaction mixture was filtered, washed with dichloromethane, and the solvent was removed by rotary evaporation, and then purified by silica gel column chromatography (the silica gel specification was 200 to 300 mesh, the mass ratio of silica gel to the product to be purified was 200:1, and the eluent was petroleum ether and ethyl acetate volume ratio is 3:1 mixed liquor), obtain targe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com