New applications of neuropeptide y
A neuropeptide, inflammatory response technology, applied in the field of medicine, can solve the problems of high incidence and mortality of heart failure, and achieve the effect of reducing myocardial infarction area, slowing myocardial remodeling, and improving cardiac dysfunction.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] 1. Experimental materials
[0067] (1) 129S2 / SvPasCrl mice: aged from 8 to 10 weeks, with a body weight of 20g to 22g; all operations on the experimental 129S2 / SvPasCrl mice were performed in accordance with the guidelines for the feeding and use of experimental animals issued by the National Institutes of Health ( National Institutes of Health publication number 85-23, revised 1996).
[0068] (2) Anesthetic and sedative drugs: ketamine (50mg / kg) and xylazine (50mg / kg).
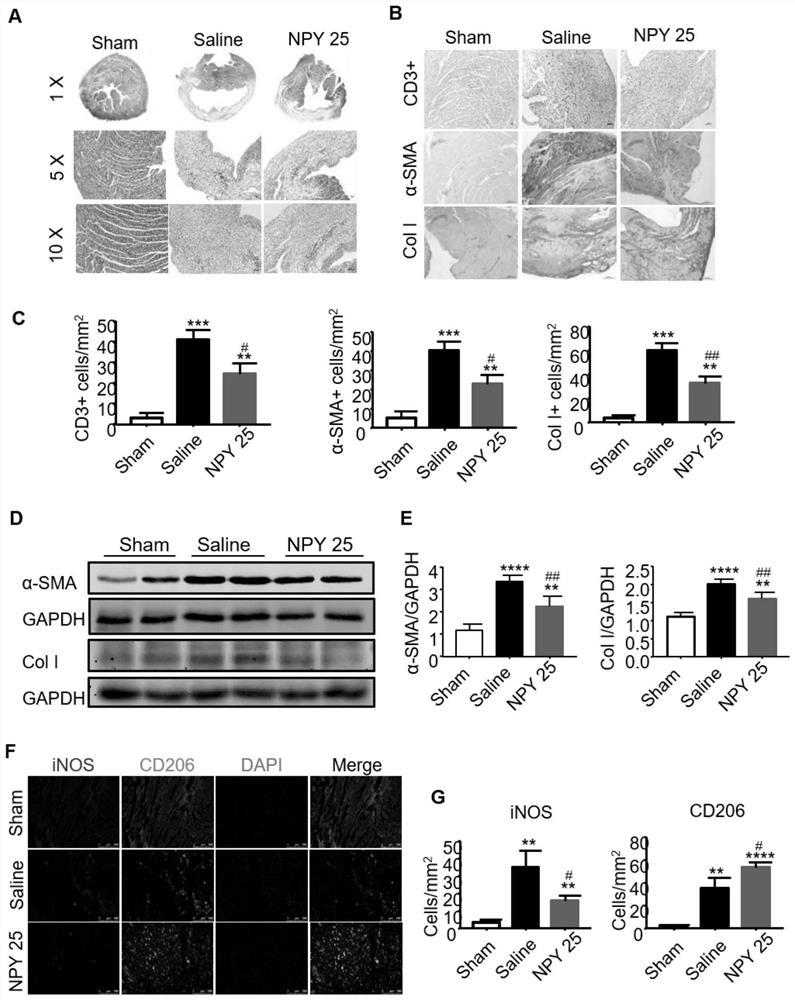
[0069] (3) Neuropeptide Y (Neuropeptide Y, NPY) powder: purchased from Sigma, USA, prepared with 0.9% by mass saline. The preparation concentration of this embodiment includes 12.5 μg / kg, 25 μg / kg, 50 μg / kg and 100 μg / kg.
[0070] 2. Experimental method
[0071] (1) Mouse anesthesia:
[0072] Take 129S2 / SvPasCrl mice, inject ketamine (50 mg / kg) and xylazine (50 mg / kg) intraperitoneally according to the weight of the mice, and anesthetize the 129S2 / SvPasCrl mice.
[0073] (2) Construction of model ...
Embodiment 2
[0089] 1. Experimental materials
[0090] (1) 129S2 / SvPasCrl mice: aged from 8 to 10 weeks, with a body weight of 20g to 22g; all operations on the experimental 129S2 / SvPasCrl mice were performed in accordance with the guidelines for the feeding and use of experimental animals issued by the National Institutes of Health ( National Institutes of Health publication number 85-23, revised 1996).
[0091] (2) Anesthetic and sedative drugs: ketamine (50mg / kg) and xylazine (50mg / kg).
[0092] (3) Neuropeptide Y (Neuropeptide Y, NPY) powder: purchased from Sigma, USA, prepared with 0.9% by mass saline. The preparation concentration of this embodiment includes 12.5 μg / kg, 25 μg / kg, 50 μg / kg and 100 μg / kg.
[0093] 2. Experimental method
[0094] (1) Mouse anesthesia:
[0095] Take 129S2 / SvPasCrl mice, inject ketamine (50 mg / kg) and xylazine (50 mg / kg) intraperitoneally according to the weight of the mice, and anesthetize the 129S2 / SvPasCrl mice.
[0096] (2) Construction of model ...
Embodiment 3
[0112] 1. Experimental materials
[0113] (1) 129S2 / SvPasCrl mice: aged from 8 to 10 weeks, with a body weight of 20g to 22g; all operations on the experimental 129S2 / SvPasCrl mice were performed in accordance with the guidelines for the feeding and use of experimental animals issued by the National Institutes of Health ( National Institutes of Health publication number 85-23, revised 1996).
[0114] (2) Anesthetic and sedative drugs: ketamine (50mg / kg) and xylazine (50mg / kg).
[0115] (3) Neuropeptide Y (Neuropeptide Y, NPY) powder: purchased from Sigma, USA, prepared with 0.9% by mass saline. The preparation concentration of this embodiment includes 12.5 μg / kg, 25 μg / kg, 50 μg / kg and 100 μg / kg.
[0116] 2. Experimental method
[0117] (1) Mouse anesthesia:
[0118] Take 129S2 / SvPasCrl mice, inject ketamine (50 mg / kg) and xylazine (50 mg / kg) intraperitoneally according to the weight of the mice, and anesthetize the 129S2 / SvPasCrl mice.
[0119] (2) Construction of model ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com