Spider polypeptide toxin with Nav1.9 specific activation effect and application of spider polypeptide toxin
An activation, spider technology, applied in the field of peptides, can solve problems such as self-harm, bone fractures, no therapeutic drugs and treatment strategies
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] HpTx1 restores pain response in Nav1.7 knockout mice
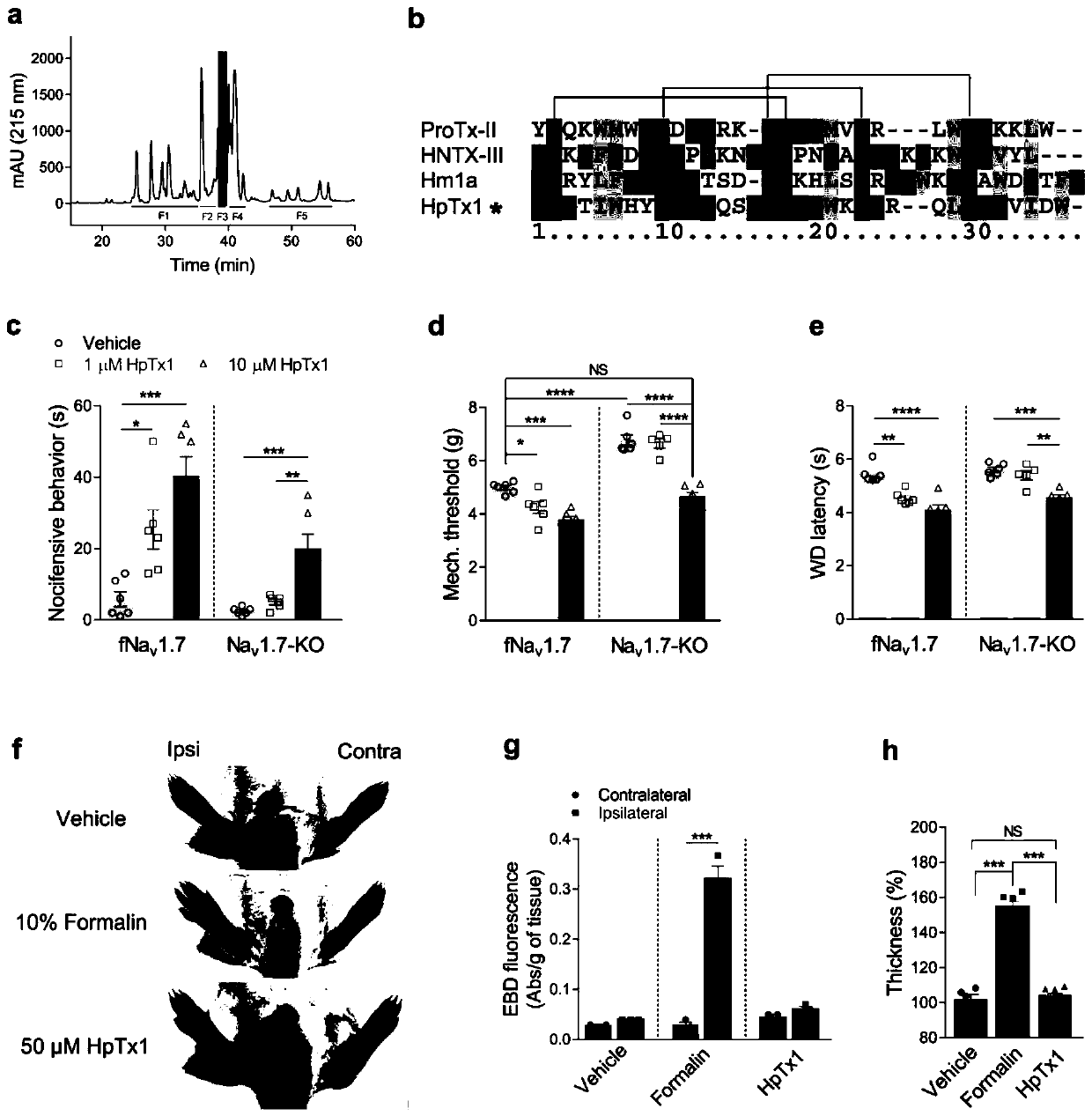
[0067] We separated the white-fronted tall spider (H. venatoria) venom by semi-preparative reversed-phase high-performance liquid chromatography (RP-HPLC). Chromatography (figure 1 Middle a panel) was purified again to obtain a polypeptide toxin HpTx1 (system name κ-sparatoxin-Hv1a), which was determined by matrix-assisted laser desorption / ionization time-of-flight mass spectrometry (MALDI-TOF MS), and its molecular weight was 3910.8Da. Sequence comparison with polypeptide toxins of known structure found that HpTx1 showed certain sequence similarities, especially the conserved cysteine pattern, which is the pattern adopted by most spider polypeptide toxins-inhibitor cystine knot ( ICK) motif ( figure 1 Middle b).
[0068] Injection of 10 μM HpTx1 into the hind paws of Nav1.7 knockout (Nav1.7-KO) mice or control littermate wild-type mice (fNav1.7) triggered strong nociceptive responses, such as licking or biting ...
Embodiment 2
[0070] HpTx1 enhances the excitability of DRG neurons in Nav1.7-KO mice
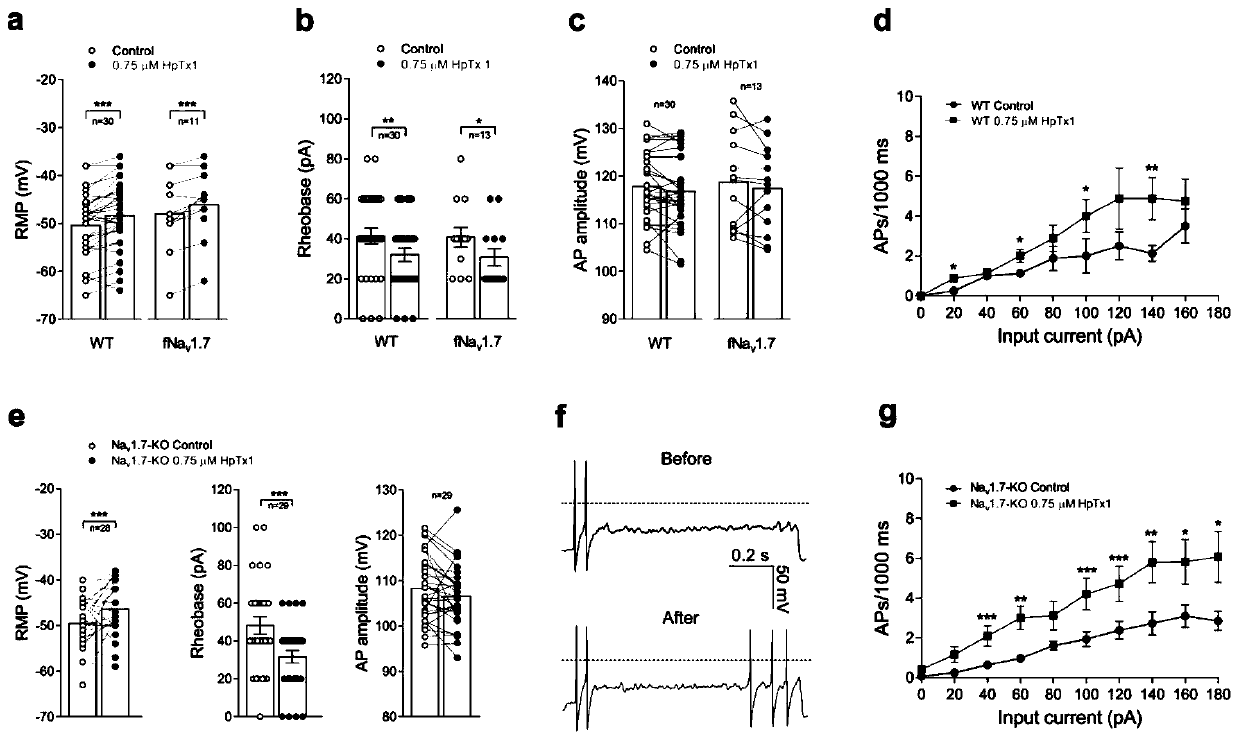
[0071] Current-clamp recordings were used to investigate whether HpTx1 directly activates sensory neurons. To detect the effect of HpTx1 on the action potential (AP) burst of small-diameter ( figure 2 As shown in panel a, 0.75 μM HpTx1 shifted the RMP by about 2.4 mV in the direction of depolarization (Table 1). At the same time, 0.75μM HpTx1 also significantly reduced the action potential current threshold, about 9.3pA ( figure 2 Middle b panel, Table 1). Of the 30 DRG neurons tested, current thresholds decreased in 15 neurons (50.0%), remained unchanged in 40%, and increased in 10% in the presence of HpTx1. However, no significant change in action potential amplitude was observed in the presence of HpTx1 ( figure 2 c, Table 1); Among the 15 neurons sensitive to HpTx1, the frequency of action potential bursts in 8 neurons was significantly increased ( figure 2 Middle d panel). Notably, the inpu...
Embodiment 3
[0077] HpTx1 inhibits Nav1.7 and activates Nav1.9
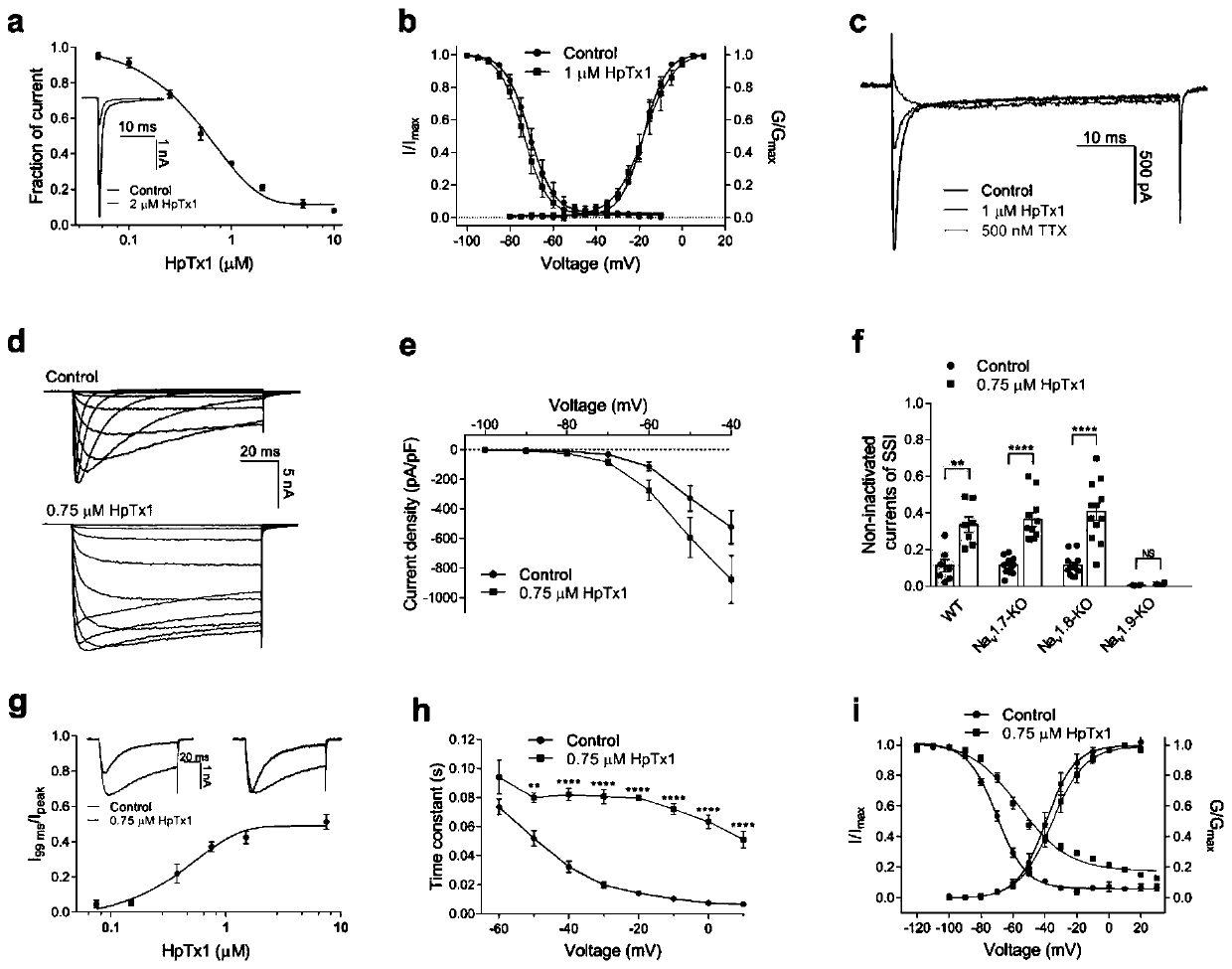
[0078] HpTx1-induced pain responses were not different between wild-type (WT) and Nav1.7-KO mice, suggesting that HpTx1-induced pain responses should not be related to Nav1.7. However, HpTx1 inhibited hNav1.7 currents expressed in HEK 293T cells, IC 50 The value is 0.51±0.12μM ( image 3 Middle panel a). However, 1 μM HpTx1 did not change the steady-state activation and inactivation curves of Nav1.7 channels ( image 3 Middle b panel, Table 2). In addition, the effect of HpTx1 on (tetrodotoxin-sensitive) TTX-S Nav channels in small-diameter DRG neurons of WT mice was detected, and it was found that 1 μM HpTx1 inhibited 62.9±7.8% of TTX-S Nav currents ( image 3 middle c). Then besides Nav1.7, TTX-S type Nav1.6 is also expressed in small diameter DRG neurons. Indeed, HpTx1 has inhibitory activity on Nav1.6 current, IC 50 The value is 5.63 ± 0.13 μM, but it is nearly 10 times weaker than that of Nav1.7. Furthermore, Vas...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com