Synthesis method of 4-chloropyrrolopyrimidine compound
A technology of chloropyrrolopyrimidine and a synthesis method, which is applied in the field of synthesis of 4-chloropyrrolopyrimidine compounds, can solve the problems of long synthesis route, high toxicity of raw materials, environmental pollution and the like, and achieves short synthesis route, low toxicity of raw materials and pollution. light effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
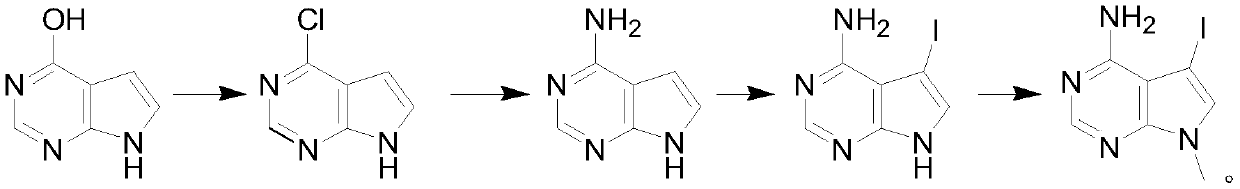
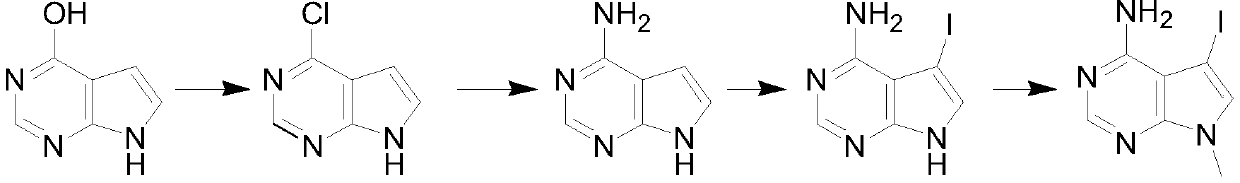
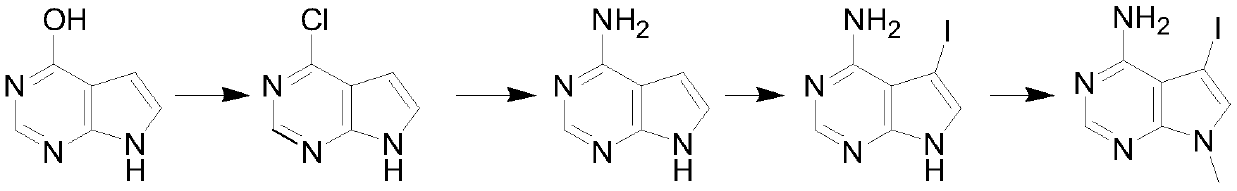
[0019] A kind of synthetic method of 4-chloropyrrolopyrimidine compound, it is characterized in that: comprise the following steps:
[0020] Step 1: Mix 4-hydroxypyrrolopyrimidine, phosphorus oxychloride and organic base in a weight ratio of 1:5-10:3-7 and carry out chlorination reaction in a certain temperature range for 2-5 hours, and depressurize the mixture after cooling Concentrate to remove excess phosphorus oxychloride, add water to quench, and then extract with an organic solvent, dry, and concentrate to obtain the product 4-chloropyrrolopyrimidine.
[0021] Step 2: Dissolve 4-chloropyrrolopyrimidine in ethanol, start to add the ethanol solution of ammonia dropwise, after adding ethanol, control the reaction at 60°C for 2-3 hours, and remove excess ethanol after the reaction to obtain 4-aminopyrrolopyrimidine pyrimidine.
[0022] Step 3: Dissolve 4-aminopyrrolopyrimidine in dichloromethane, add iodosuccinimide in batches at room temperature, and stir and react at room...
specific Embodiment
[0030] (1) After mixing 100g of 4-hydroxypyrrolopyrimidine, 400g of phosphorus oxychloride and 500g of triethylamine and diisopropylethylamine, the temperature is controlled at 50°C, and the chlorination reaction is carried out for 3 hours, and the mixture is cooled Concentrate under reduced pressure to remove excess phosphorus oxychloride, add water to quench, then extract with organic solvent, dry, and concentrate to obtain the product 4-chloropyrrolopyrimidine.
[0031] (2) Dissolve the generated 4-chloropyrrolopyrimidine in 500g of ethanol, start to add the ethanol solution of ammonia dropwise, and keep the reaction at 60°C for 2 hours. The excess ethanol was evaporated to obtain 4-aminopyrrolopyrimidine.
[0032] (3) Dissolving 4-aminopyrrolopyrimidine in 200 g of dichloromethane liquid, adding iodosuccinimide in batches at room temperature, and stirring and reacting at room temperature for 13 hours, after the reaction was completed, the solid insolubles were removed by f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com