Seven terpenoids from euonymus verrucosus as well as preparation method and application thereof
A compound and technology of the genus Euonymus, which is applied to the field of terpenoids in Euonymus vulgaris and their preparation fields, can solve the problems of tissue around cells and cell damage and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
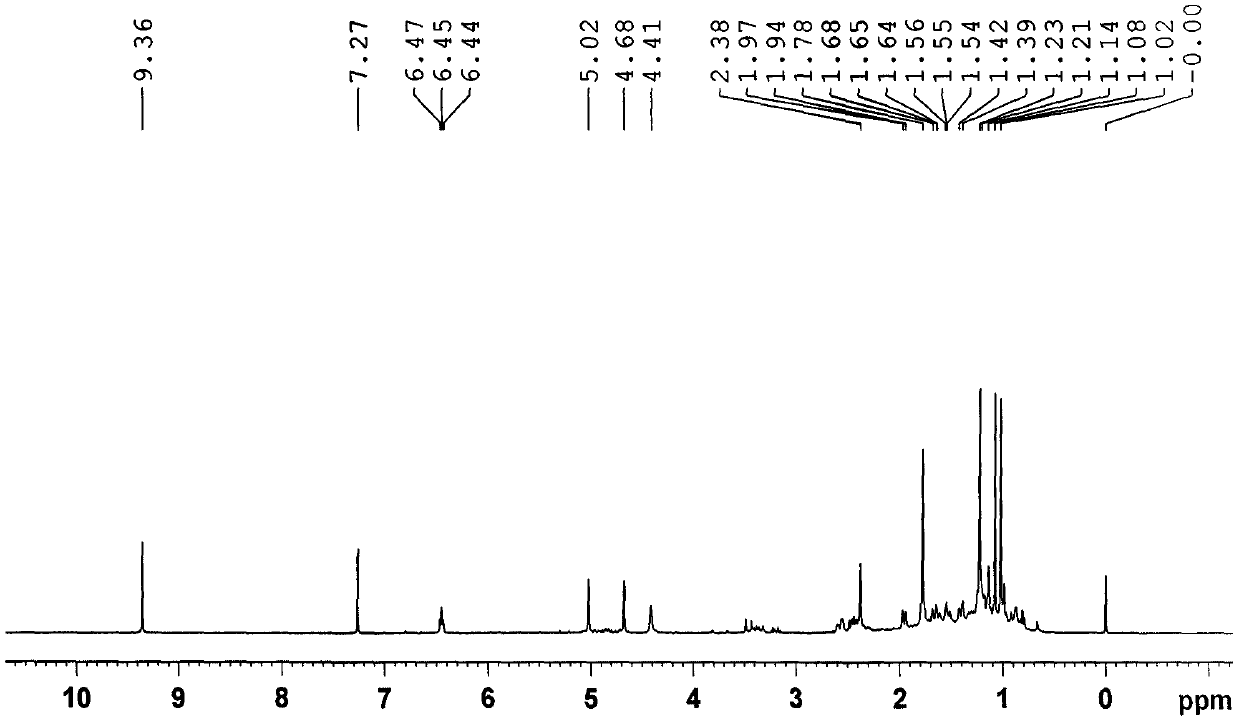
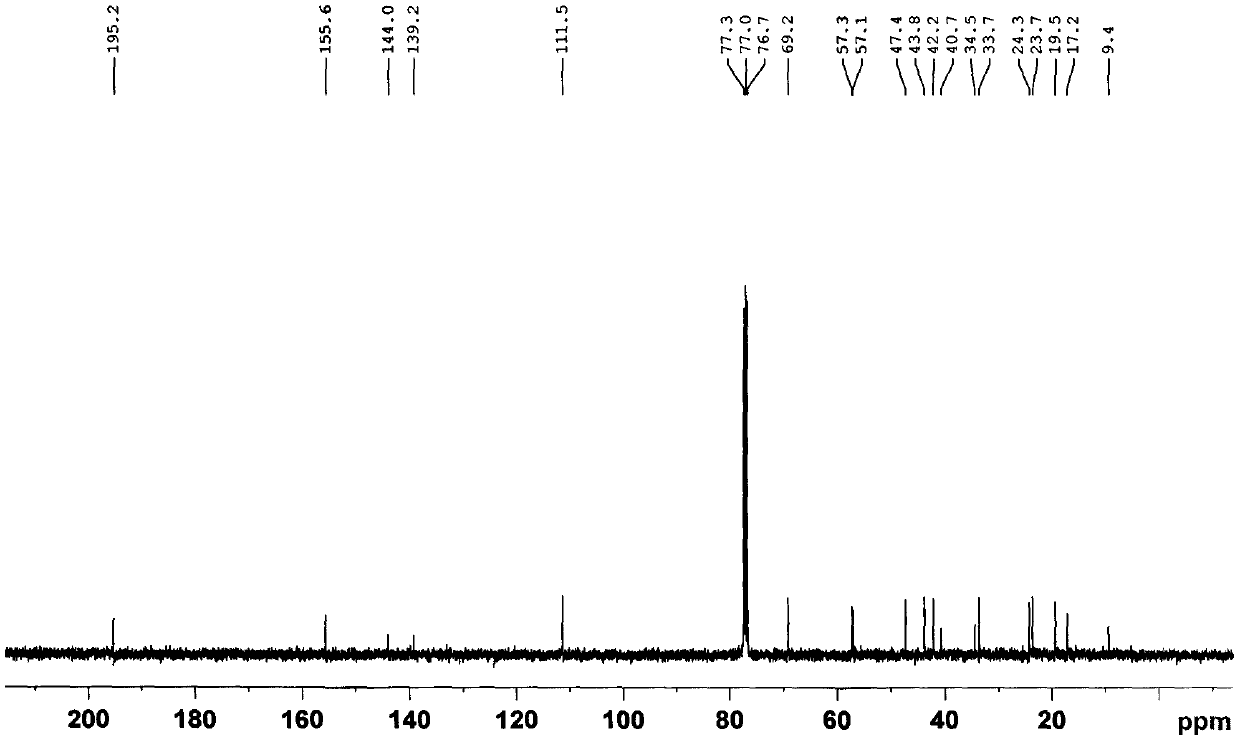
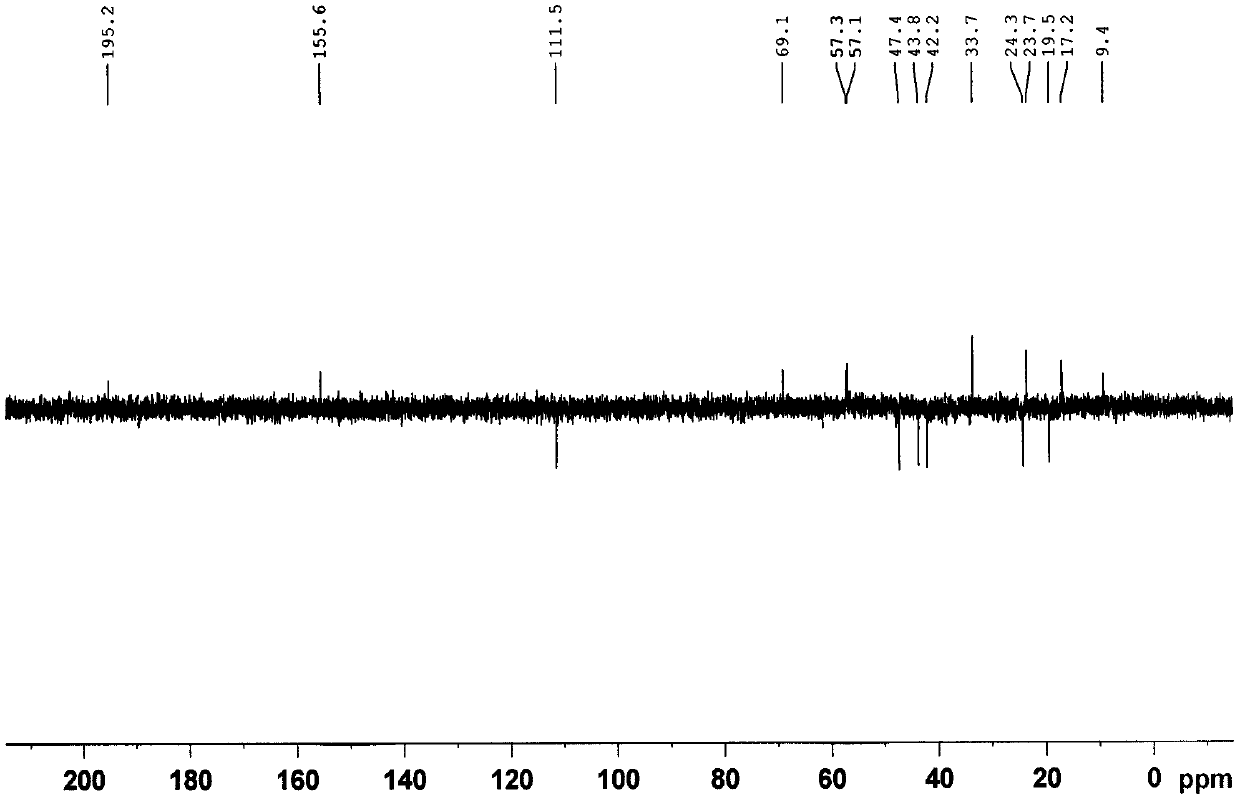
[0053] (1) 10.5 kg of Euonymus twigs with less flower tumors were extracted with methanol for 3 times under reflux (amount of 3 × 60 L), and the extract was recovered under reduced pressure to obtain a crude extract;
[0054] (2) The methanol extract obtained in step (1) is added with water to make a suspension, and extracted with ethyl acetate to obtain the ethyl acetate extract;
[0055] (3) Step (2) is separated by silica gel column chromatography, followed by petroleum ether: acetone 100:0, 100:2, 100:4, 100:6, 100:9, 100:13, 100:30, 100: 40 elution;
[0056](3) Petroleum ether obtained in the above step (2): 100:2~100:13 fractions of acetone are separated by medium pressure liquid chromatography (MPLC), using methanol / water 6:4~9:1 as mobile phase gradient Elution;
[0057] (4) The methanol / water (6:4~9:1) fraction obtained in the above step (3) was separated by HPLC-RI, and the new compound 1 was obtained by eluting with methanol / water 60:40~90:10 as the mobile phase ...
Embodiment 2
[0078] (1) 8.0 kg of euonymus twigs with less flower tumors were extracted with ethanol 3 times (amount of 3 × 48 L), and the extract was recovered under reduced pressure to obtain a crude extract;
[0079] (2) The ethanol extract obtained in step (1) is added with water to make a suspension, and extracted with ethyl acetate to obtain the ethyl acetate extract;
[0080] (3) Step (2) is separated by silica gel column chromatography, followed by petroleum ether: ethyl acetate 100:0, 100:2, 100:4, 100:6, 100:9, 100:15, 100:28, 100:42 elution;
[0081] (3) Petroleum ether obtained in the above step (2): ethyl acetate 100:2~100:15 fractions are separated by medium pressure liquid chromatography (MPLC), using methanol / water 6:4~9:1 as the flow Phase gradient elution;
[0082] (4) The methanol / water (6:4~9:1) fraction obtained in the above step (3) was separated by HPLC-RI, and the new compound 1 was obtained by eluting with methanol / water 60:40~90:10 as the mobile phase (yield 0....
Embodiment 3
[0085] (1) 8.0 kg of Euonymus twigs with less flower tumors were extracted 3 times with acetone (amount of 3 × 48 L), and the extract was recovered under reduced pressure to obtain a crude extract;
[0086] (2) The acetone extract obtained in step (1) is added with water to make a suspension, and extracted with dichloromethane to obtain the dichloromethane extract;
[0087] (3) Step (2) is separated by silica gel column chromatography, followed by petroleum ether: acetone 100:0, 100:2, 100:4, 100:6, 100:9, 100:13, 100:28, 100: 40 elution;
[0088] (3) Petroleum ether obtained in the above step (2): 100:2~100:13 fractions of acetone are separated by medium pressure liquid chromatography (MPLC), using methanol / water 6:4~9:1 as mobile phase gradient Elution;
[0089] (4) The methanol / water (6:4~9:1) fraction obtained in the above step (3) was separated by HPLC-RI, and the new compound 1 was obtained by eluting with acetonitrile / water 60:40~90:10 as mobile phase (yield 0.002%),...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com