Predicting responses to immunotherapy
A technology for response and therapy, applied in flow cytometry, cell sorting and related technologies, which can solve problems such as expensive
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0106] Preparation of Positive Reaction Control
[0107] The invention may include the additional step of evaluating one or more organs or tissues of an individual who has received an immunomodulator to determine regression of the tumor in said individual, thereby determining whether said individual is suitable to provide a positive response control. For example, where the method is used to predict the likelihood of a complete response, the step includes assessing whether a candidate individual for providing a positive response control has responded completely or partially, or whether there is any response at all. In one embodiment, the step utilizes radiographic imaging to determine the location and volume of each of a plurality of tumor foci in the subject following treatment with the immunomodulator. For example, this may involve a three-dimensional radiological image of the subject showing the geographic location of each of the plurality of tumor lesions. Non-limiting e...
Embodiment 1
[0181] Example 1: Determining the distribution of cells in a sample
[0182] Peripheral blood samples are obtained using venipuncture and the blood is collected into tubes that prevent clotting (eg, tubes containing EDTA or heparin). The leukocytes are then immediately prepared for cell counting, or frozen for long-term storage, such as at -80 or in liquid nitrogen. Cryopreserved leukocytes were thawed prior to analysis using conventional techniques.
[0183] The distribution of cells is determined using techniques that identify the expression of predetermined proteins or transcripts within each individual cell in the sample. Typically, cells are treated with a mixture of 10 to 40 antibodies, each specific for a single cellular protein and labeled with a unique metal isotope or fluorescent dye, allowing the treated cells to be analyzed using mass cytometry or fluorescent cytometry, respectively. Samples are analyzed.
[0184] In the examples provided, cells were treated w...
Embodiment 2
[0226] Example 2: Comparing the distribution of cell populations in different individuals.
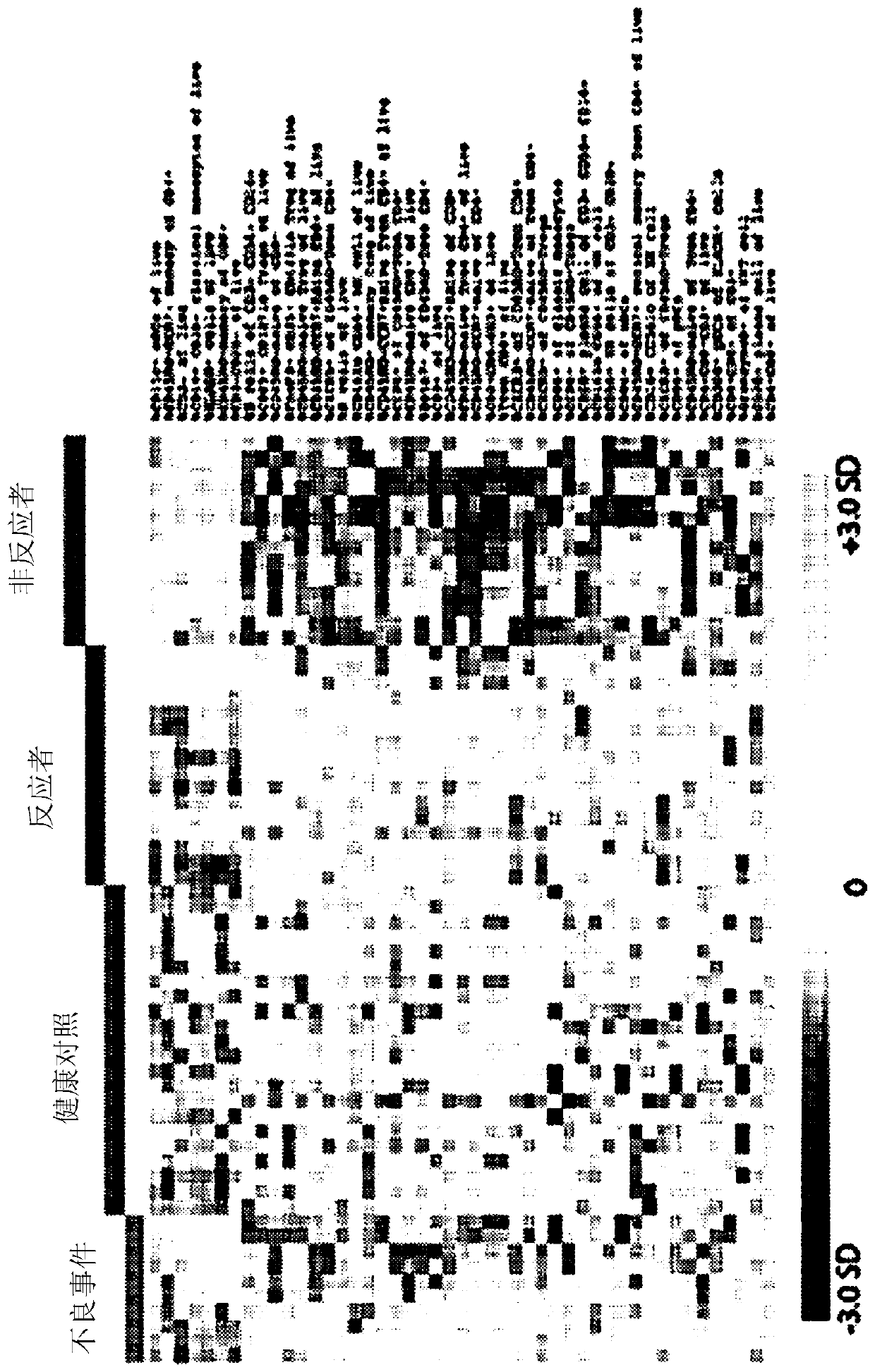
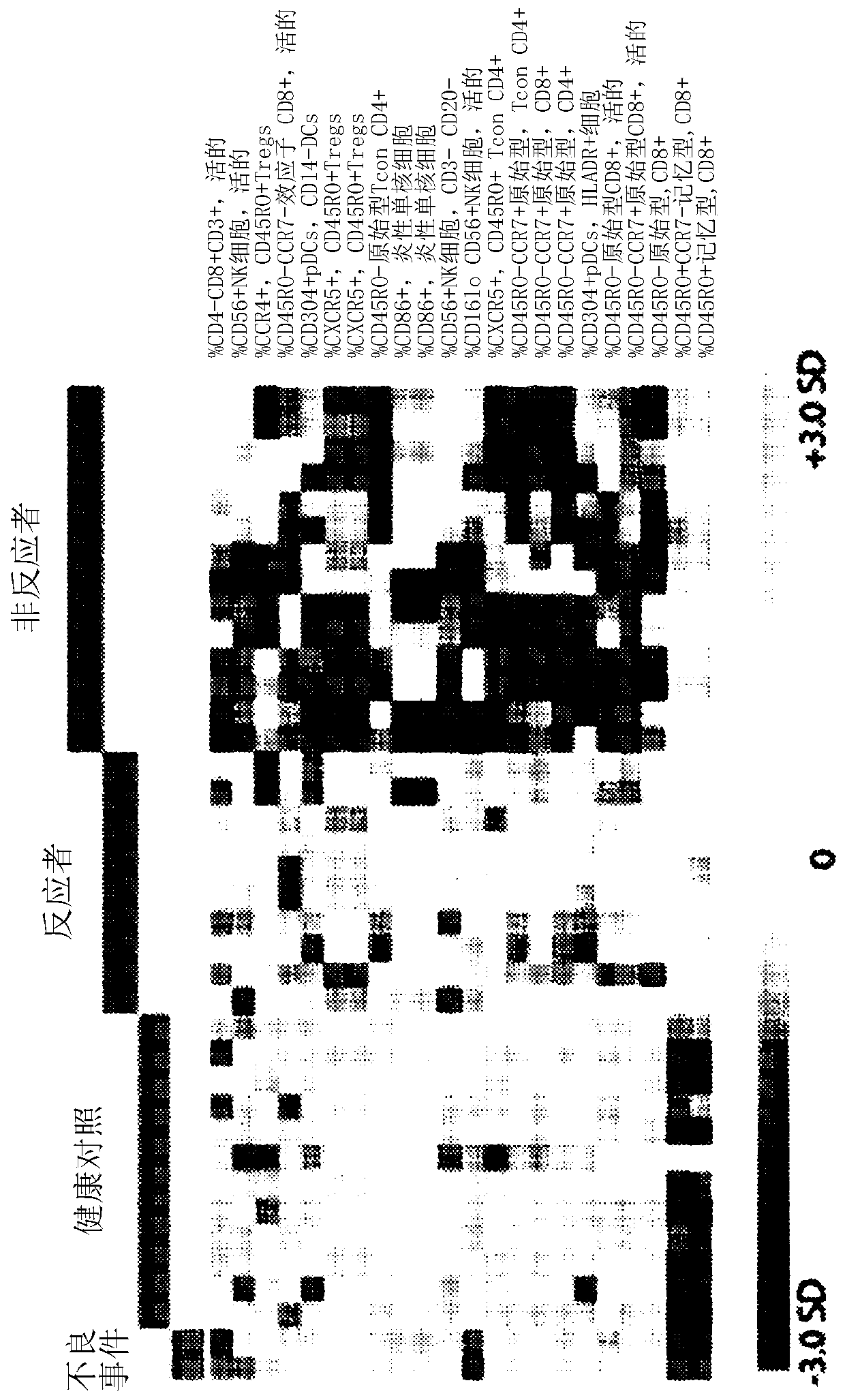
[0227] This can be achieved with many statistical techniques including machine learning. A technique based on microarray analysis is presented here.
[0228] Load the percentage data for all individuals to be compared into a microarray analysis software package such as MeV. Row normalization is performed so that the relative weight of each cell population within the dataset is equal to the relative weight of every other population.
[0229] The graph output after row normalization is as follows figure 1 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com