Diphyllin ether derivative and preparation method and application thereof
A technology of lotus leaf ether and derivatives, which is applied in the fields of medicinal chemistry and pharmacology, can solve the problems of poor metabolic stability of glycosidic bonds, hydrolysis inactivation of glycosidase, complicated chemical synthesis, etc., and achieves the effect of strong tumor cell proliferation inhibition activity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
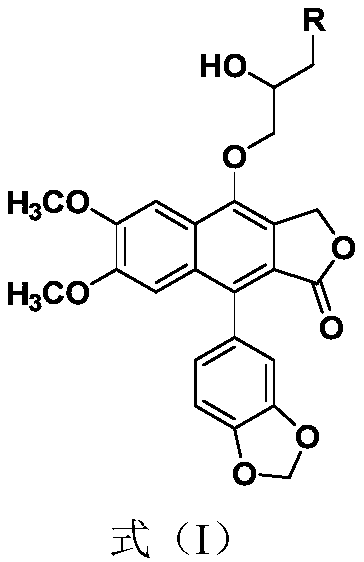
[0044] Dissolve 190mg (0.5mmol) of kaempferol in 6mL of DMF, add 343mg (2.5mmol) of epibromohydrin and 414mg (3.0mmol) of anhydrous potassium carbonate, and react at 60°C for 2h. TLC detected the end of the reaction, added 50mL of ethyl acetate to dilute, then washed with water and saturated brine successively, MgSO 4 Drying, drying under reduced pressure, column chromatography (prtroleum ether:EtOAc=1:1, R f =0.3) 174 mg of light yellow solid was obtained, the yield was 80%. Dissolve the light yellow solid in 20 mL of MeOH, add 88 mg (1.2 mmol) of n-butylamine, react at 65 ° C for 3 h, TLC monitors the end of the reaction, MgSO 4 Drying, drying under reduced pressure, column chromatography (DCM:MeOH=20:1, R f = 0.3) 124 mg of white powder 4-O-(3'-butylamino-2'-hydroxypropyl)-baciferin (2a) was obtained with a yield of 61%.
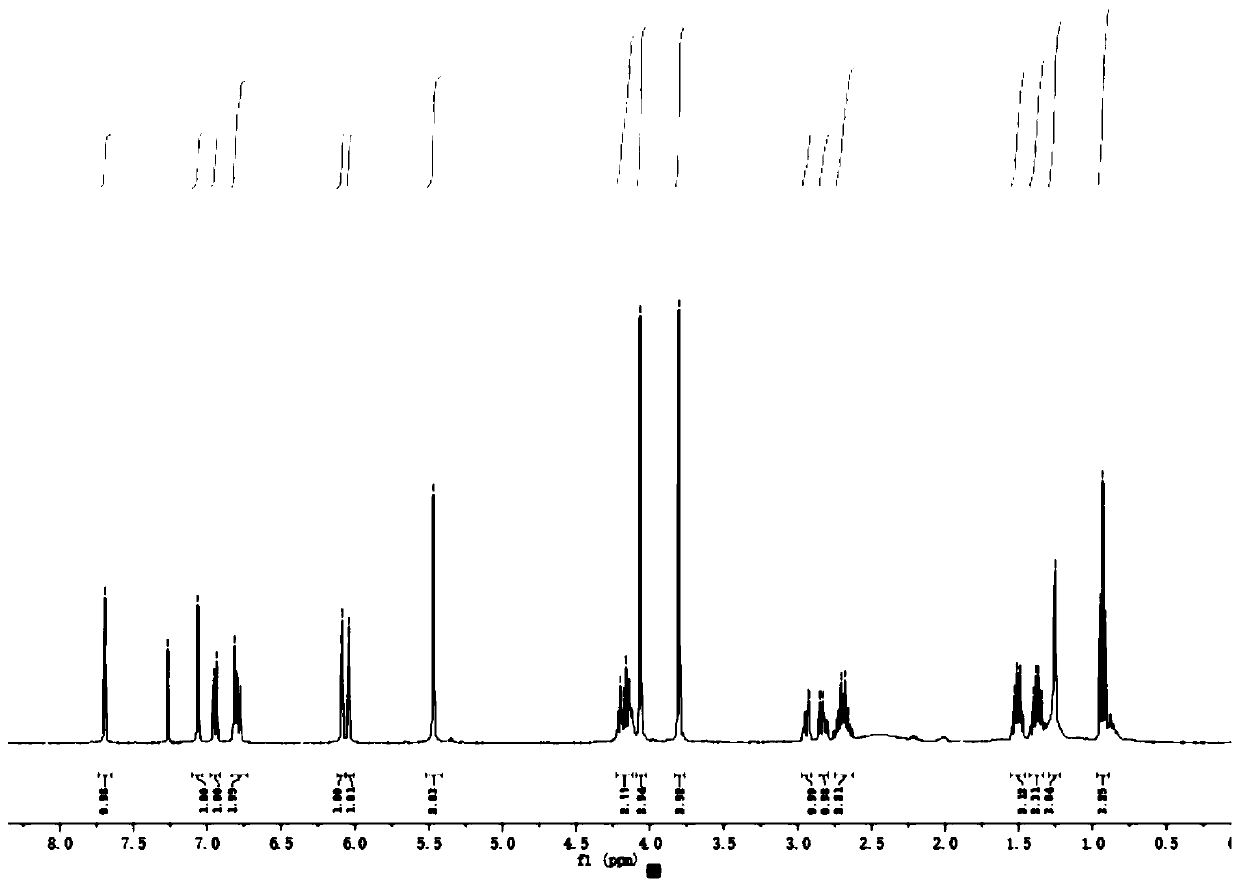
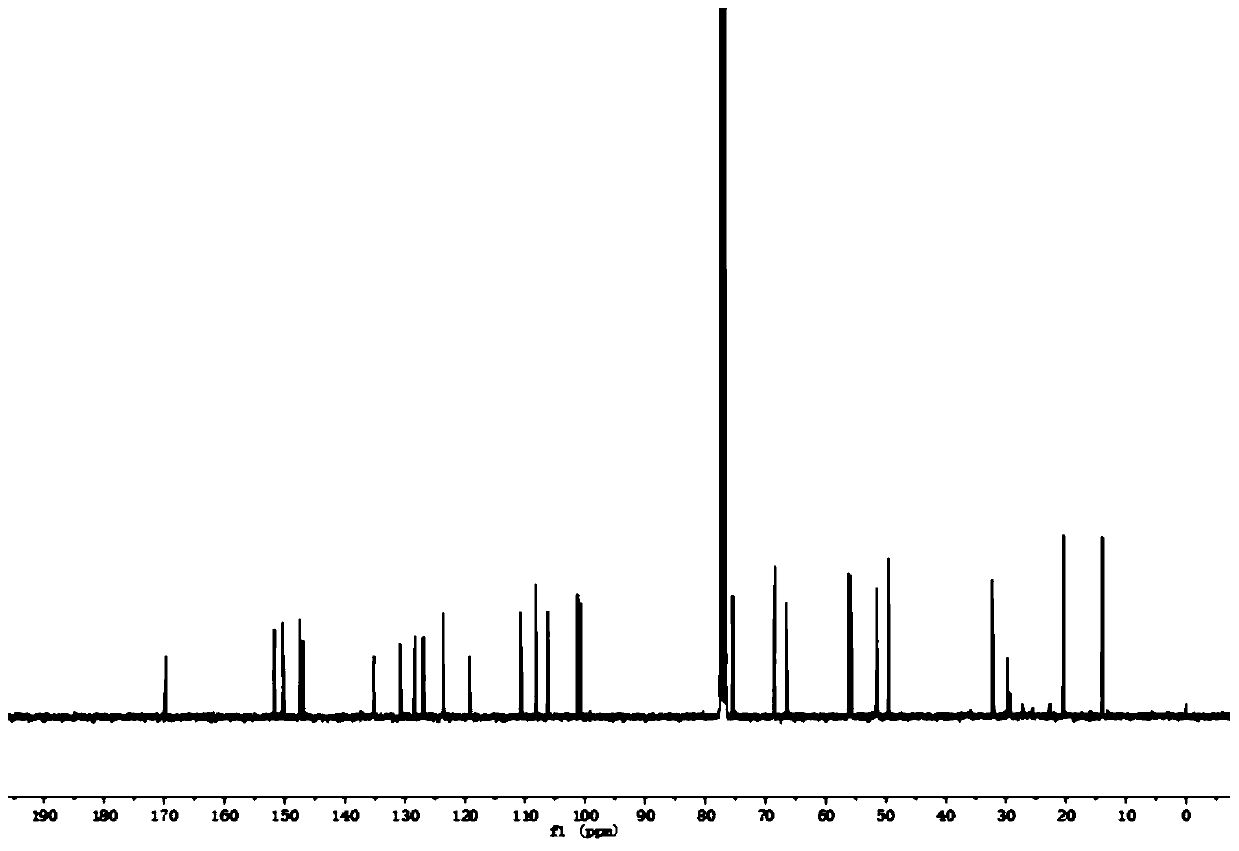
[0045] For NMR detection of 4-O-(3'-butylamino-2'-hydroxypropyl)-kaempferol (2a), see figure 1 and figure 2 , resulting in: 1 H NMR (400MHz, CDCl ...
Embodiment 2
[0047] According to the method of Example 1 above, 88mg (1.2mmol) of n-butylamine was replaced by 128mg (1.2mmol) of benzylamine to prepare 4-O-(3'-benzylamino-2'-hydroxypropyl) -Nuciferin (2b), the physicochemical data of this compound are:
[0048] 2b: 1 H NMR (400MHz, CDCl 3 )δ: 7.64(s,1H,ArH),7.39-7.30(m,5H,ArH),7.06(s,1H,ArH),6.95(d,J=7.9Hz,1H,ArH),6.85-6.75( m,2H,ArH),6.06(dd,J=19.8,1.4Hz,2H,OCH 2 O),5.44(s,2H,ArCH 2 O), 4.24–4.06 (m, 3H, CH 2 ,CH),4.01(s,3H,OCH 3 ), 3.87 (d, J=5.3Hz, 2H, CH 2 ),3.80(s,3H,OCH 3 ),3.04-2.72(m,2H,CH 2 ). 13 C NMR (100MHz, CDCl 3 )δ: 169.7, 151.7, 150.3, 147.5, 146.7, 139.7, 135.1, 130.7, 128.6, 128.4, 128.1, 127.4, 126.9, 126.7, 123.6, 119.2, 110.8, 108.2, 1066.2, 1031.2, 8.5, 10 ,56.1,55.8,53.8,50.9.HRMS(ESI):m / z calcd for C 31 h 30 NO 8 :544.1964; found: 544.1961[M+H] + .
Embodiment 3
[0050] According to the method of Example 1 above, 88 mg (1.2 mmol) of n-butylamine was replaced with 112 mg (1.2 mmol) of aniline to prepare 4-O-(3'-anilino-2'-hydroxypropyl)-shank Nuciferin (2c), the physicochemical data of this compound are:
[0051] 2c: 1 H NMR (400MHz, CDCl 3 )δ: 7.60 (s, 1H, ArH), 7.25-7.13 (m, 2H, ArH), 7.04 (s, 1H, ArH), 6.91 (d, J=7.9Hz, 1H, ArH), 6.83-6.62 ( m,5H,ArH),6.17-5.88(m,2H,OCH 2 O),5.41(s,2H,ArCH 2 O), 4.35(td, J=6.8, 3.1Hz, 1H, CH), 3.99(s, 3H, OCH 3 ),4.29-4.20(m,2H,CH 2 ),3.79(s,3H,OCH 3 ),3.58-3.31(m,2H,NCH 2 ). 13 C NMR (100MHz, CDCl 3 )δ: 169.9, 151.8, 150.3, 148.0, 147.5, 146.5, 135.2, 130.7, 129.4, 128.3, 126.5, 123.6, 119.1, 118.4, 113.3, 110.7, 108.2, 106.3, 1561.3, 100.4, 9.4 ,55.9,46.7.HRMS(ESI):m / zcalcd for C 30 h 28 NO 8 :530.1807; found: 530.1804[M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com