Environment-friendly plant bactericide
A plant fungicide, an environmentally friendly technology, applied in the field of new compounds, can solve the problems of no research and development reports on activity, and achieve excellent biological activity, novel structure, and good bactericidal effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
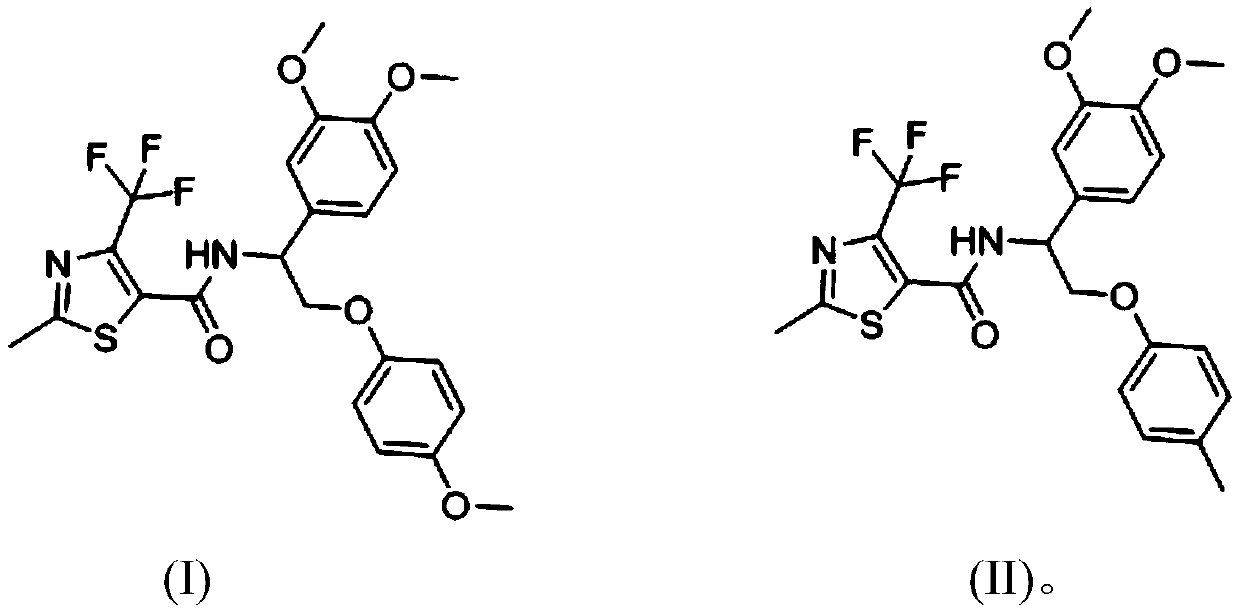
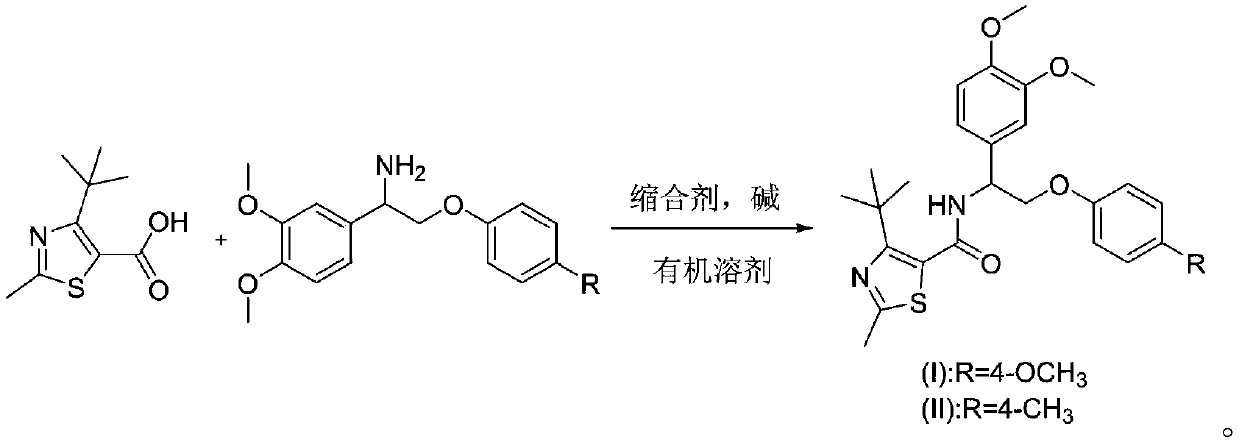
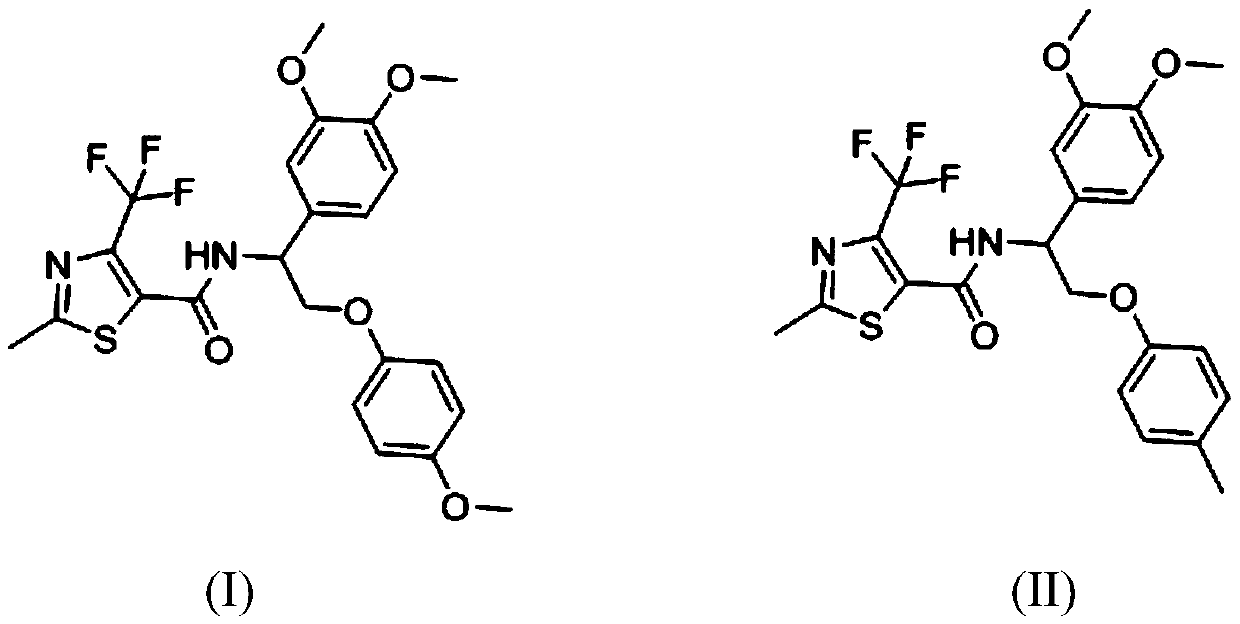
[0030] N-(1-(3,4-dimethoxyphenyl)-2-(4-methoxyphenyl)ethyl)-2-methyl-4-(trifluoromethyl)thiazole-5- Preparation of formamide
[0031] 2-Methyl-4-trifluoromethyl-5-thiazolecarboxylic acid (0.211g, 1.0mmol), 1-(3,4-dimethoxyphenyl)-2-(4-methoxyphenoxy Base) ethylamine (0.303g, 1.0mmol) was dissolved in dichloromethane, added triethylamine, then added EDCI (0.38g, 2.0mmol), HOBt (0.26g, 2.0mmol), 50 ℃ for 8 hours, TLC The detection reaction was complete, the organic layer was washed three times with water, washed once with saturated brine, dried over anhydrous sodium sulfate, and desolvated, and separated on a silica gel chromatography column using petroleum ether / EA (4:1) solvent to obtain a white solid that is N- (1-(3,4-Dimethoxyphenyl)-2-(4-methoxyphenyl)ethyl)-2-methyl-4-(trifluoromethyl)thiazole-5-carboxamide , m.p.122-125°C, yield 78%.
[0032] 1HNMR (400MHz, CDCl3) δ: 6.82–7.03 (m, 7H, phenyl), 5.35–5.38 (m, 1H, NHCH), 4.19-4.29 (m, 2H, OCH2), 3.87 (s, 6H, 2xOCH3) 3.76...
Embodiment 2
[0034] N-(1-(3,4-dimethoxyphenyl)-2-(4-methylphenyl)ethyl)-2-methyl-4-(trifluoromethyl)thiazole-5-methanol Preparation of amides
[0035] 2-Methyl-4-trifluoromethyl-5-thiazolecarboxylic acid (0.224g, 1.0mmol), 1-(3,4-dimethoxyphenyl)-2-(4-methylphenoxy ) Ethylamine (0.353g, 1.0mmol) was dissolved in N,N-dimethylformamide, triethylamine was added, then HOBt (0.653g, 2.0mmol) was added, and the reaction was carried out at 50°C for 8 hours. TLC detected that the reaction was complete. The organic layer was washed three times with water, washed once with saturated brine, dried over anhydrous sodium sulfate, and precipitated, and separated on a silica gel chromatography column using petroleum ether / EA (4:1) solvent to obtain an off-white solid, namely N-(1 -(3,4-Dimethoxyphenyl)-2-(4-methylphenyl)ethyl)-2-methyl-4-(trifluoromethyl)thiazole-5-carboxamide, m.p.129 -131°C, yield 83%.
[0036] 1HNMR (400MHz, CDCl3) δ: 7.39 (d, J = 8.0Hz, 2H.phenyl), 6.78-6.98 (m, 5H, phenyl), 5.38-5...
experiment example
[0038] (1) Test materials
[0039] The tested strains of Sclerotinia sclerotiorum were collected, isolated, cultivated and preserved by the Pesticide Science Teaching and Research Office of the Plant Protection College of Shenyang Agricultural University. The control drug chlorothalonil (chlorothalonil) original drug was provided by the Department of Pesticide Science, School of Plant Protection, Shenyang Agricultural University, and the contents were 95%.
[0040]Mycelia growth rate method First prepare the test agent with acetone to make 5000, 2500, 1250, 625.0, 312.5mg / L liquid medicine, then dilute 100 times with potato dextrose agar (PDA) medium to make toxic medium . Set the general sieve concentration as 50 mg / L, and the gradient concentration as 50, 12.5, 6.25, 3.125 mg / L. Use a puncher with a diameter of 6mm to make bacterial blocks from each pathogenic bacteria grown on the PDA medium, inoculate it on the poisonous PDA medium, culture it at 23°C±1°C for 3-5 days, a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com