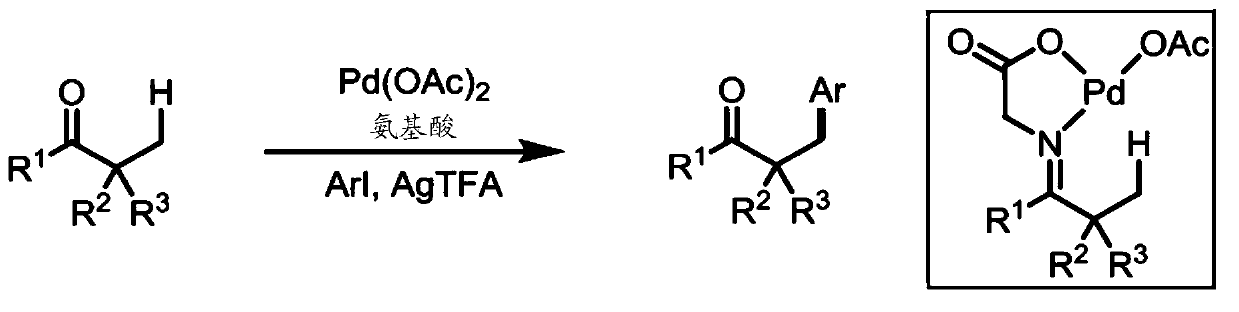
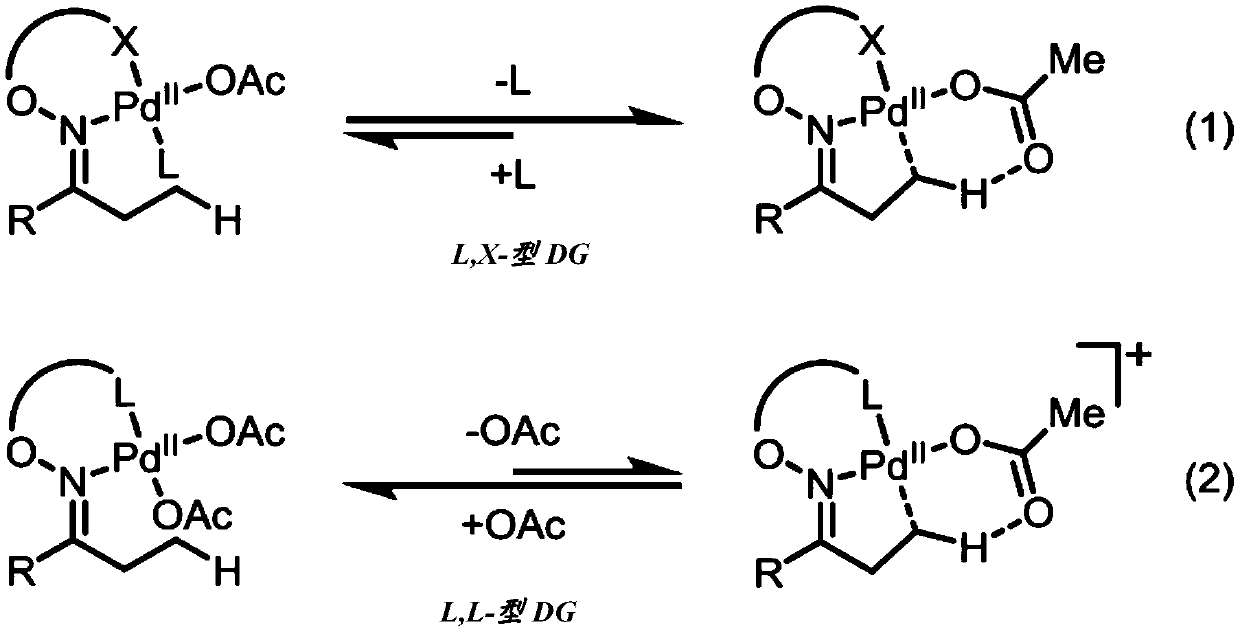
Directed beta-c(SP3)-h iodination and arylation of ketones
An iodination, oxime-based technology, applied in the field of directional β-C(sp3)-H iodination and arylation of ketones, which can solve incompatibility, substrate range and transformation limitations, interference with coupling agents and catalysts, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0068] General information
[0069] Ketones were obtained from commercial sources or synthesized according to literature procedures and used to prepare the corresponding substrates. Aminooxyacetic acid hemihydrochloride was obtained from Combi-Blocks. I 2 Obtained from TCI. PhI(OAc) 2 Obtained from Sigma-Aldrich. Solvents were obtained from Sigma-Aldrich, Alfa-Aesar and Acros and were used without further purification. Analytical thin layer chromatography was performed on 0.25 mm silica gel 60-F254. Visualization was performed using UV light and Vogel's permanganate. Recorded on Bruker AMX-400 instrument (400MHz) or Bruker DRX-600 instrument (600MHz) 1 H NMR. Chemical shifts are expressed in parts per million (ppm) relative to 0.0 ppm of tetramethylsilane. The following abbreviations (or combinations thereof) are used to explain multiplicity: s=singlet, d=doublet, t=triplet, q=quartet, m=multiplet, br=broad. The coupling constant J is expressed in Hertz units (Hz). ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com