A kind of reagent for distinguishing and detecting thiophenol and its synthesis method and application
A synthesis method and detection reagent technology, which are applied in the synthesis method of benzothiazole derivatives and its application in distinguishing and detecting thiophenols, can solve the problems of central nervous system damage, hindlimb paralysis, coma, etc., and achieve response time Short, strong specificity, obvious effect of detection signal
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
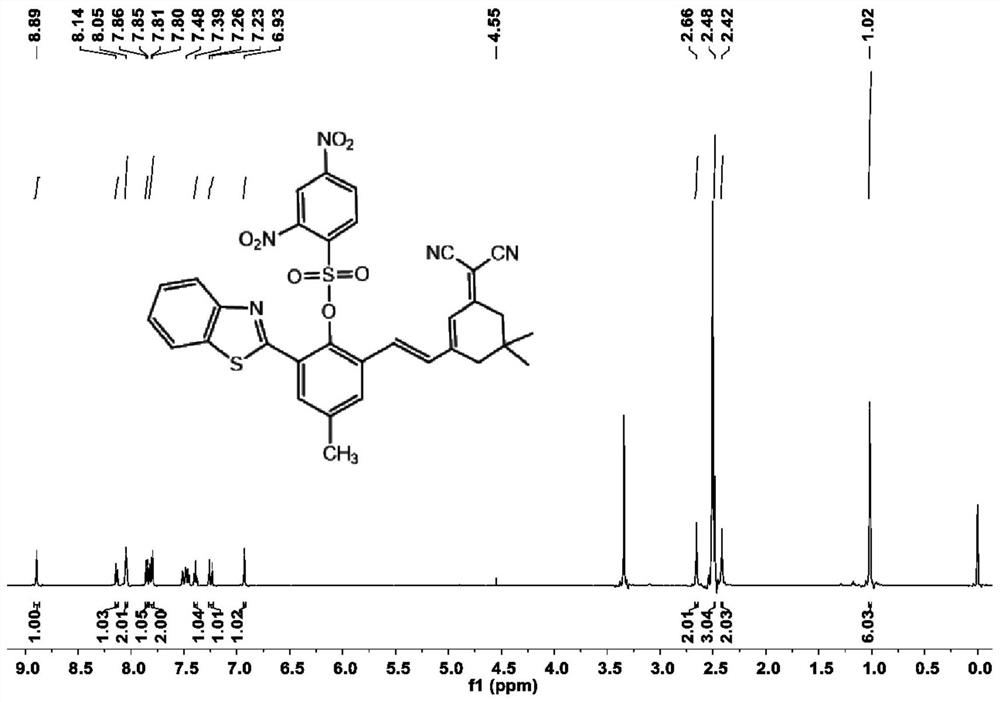
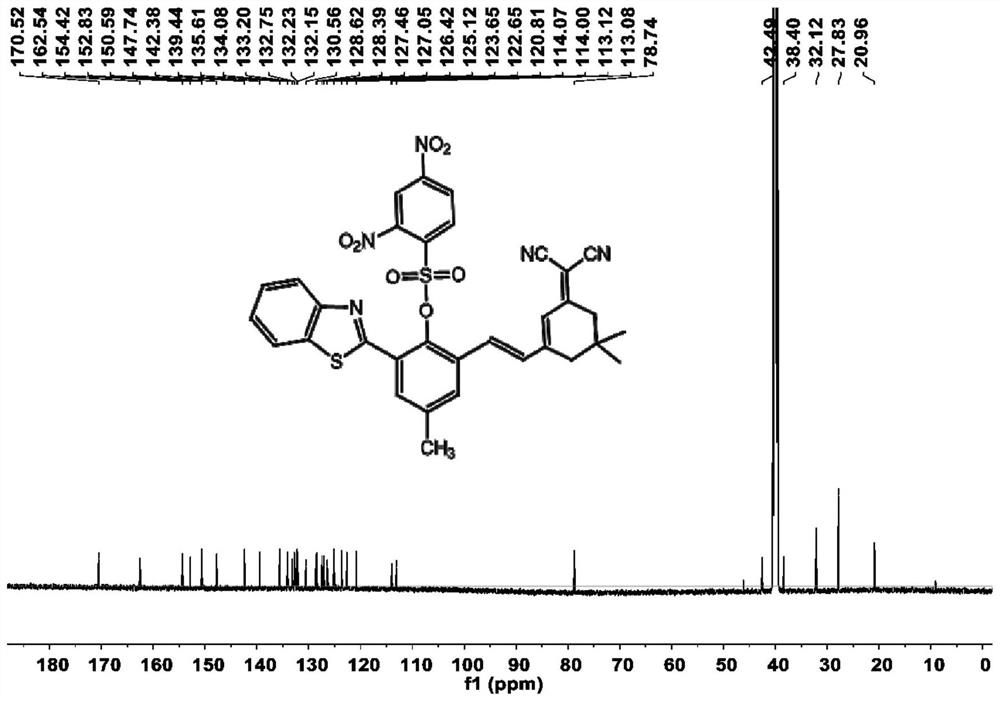
[0032] Preparation and characterization of SYN
[0033] In a 50 mL round bottom flask, 2-aminothiophenol (5 mmol, 0.625 g) and 5-methylsalicylaldehyde (5 mmol, 0.681 g) were added to 15 mL of DMSO, and silver nitrate (0.05 mmol, 0.009 g ) with stirring, the mixture was reacted at room temperature in the dark for 2 hours. After the reaction was completed, it was diluted 3 times with dichloromethane (50 mL), washed with brine, the organic phases were combined, dried over anhydrous sodium sulfate, concentrated and purified by column chromatography (ethyl acetate / petroleum ether=1:6 ) to obtain the compound as a pale yellow solid (0.893 g, 74.07%), which is 2-benzothiazol-2-yl-4-methylphenol. 1 H NMR (600MHz, Chloroform-d) δ8.01(d, J=8.1Hz, 1H), 7.93(d, J=7.9Hz, 1H), 7.56-7.48(m, 2H), 7.43(t, J= 7.5Hz, 1H), 7.21(d, J=8.3Hz, 1H), 7.04(d, J=8.4Hz, 1H), 2.37(s, 3H). 13 C NMR (150MHz, Chloroform-d) δ 169.41, 155.79, 151.84, 133.79, 132.60, 128.72, 128.34, 126.67, 125.47, 122.12, 12...
Embodiment 2
[0038] Prepare CH 3 OH / CH 3 CN / Hepes (10mM) (1:1:1, v / v / v, pH=7.4) solution, prepare 2mMSYN DMSO solution, prepare 0.2mM p-methylthiophenol acetonitrile solution; take 2mL CH 3 OH / CH 3 CN / Hepes (1:1:1, v / v / v, pH=7.4) solution and 10 μL SYN DMSO solution were added to a fluorescent cuvette, and the aqueous solution of p-methylthiophenol was gradually added with a small amount of Add the sample device into the cuvette, and detect it on the fluorescence spectrophotometer while adding the sample. With the addition of p-methylthiophenol, the fluorescence intensity at 686nm gradually increases. Fluorescence emission map see Figure 4 .
Embodiment 3
[0040] Prepare CH 3 OH / CH 3 CN / Hepes (10mM) (1:1:1, v / v / v, pH=7.4) solution, prepare 2mMSYN DMSO solution, prepare 2mM p-methylthiophenol acetonitrile solution; in the fluorescence cuvette, each Add 2 mL of CH 3 OH / CH 3 CN / Hepes (1:1:1, v / v / v, pH=7.4) solution and 10 μL SYN DMSO solution, and then add 10 times the equivalent of other analytes (1) Cys, (2) Hcy, (3) GSH,(4)SO 3 2- , (5)S 2- , (6)F - , (7)Cl - , (8)OAc - , (9)I - , (10) aniline, (11) aqueous solution of phenol, and acetonitrile solution of 2 times equivalent thiophenol homologue (12) 4-aminothiophenol (20 μ M), (13) thiophenol (20 μ M) and (14 ) 4-methylthiophenol (20 μM). Detect on a fluorescence spectrophotometer, and draw a histogram of fluorescence intensity at 686nm corresponding to different analytes (see Figure 5 ). p-cresol, p-amino-thiophenol, and thiophenol can significantly increase the fluorescence intensity of the detection system at 686nm, and other analytes basically do not cause chan...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com