De-methylated anethol trisulfide derivative as well as preparation method and application thereof
A technology of medicine and preparation, which is applied in the field of demethylanisyl trisulfide derivatives and its preparation, can solve the problems of survivors of cardiovascular and cerebrovascular accidents that they cannot fully take care of themselves, and achieve good application prospects and good water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Embodiment 1, the preparation of demethyl anetellityl trisulfide (ADT)
[0043]
[0044] Mix 20 grams of trithiopyridine (M1) and 58 grams of anhydrous pyridine hydrochloride evenly, under nitrogen protection, slowly heat to 220 degrees Celsius, all the solids dissolve into a homogeneous system, keep stirring at 220 degrees Celsius for 30 minutes, and naturally cool to room temperature. Add 200ml of ethyl acetate and 200ml of water, heat to 50 degrees Celsius and stir for 30 minutes, filter to remove insoluble matter, and the obtained filtrate is allowed to stand for stratification, take the ethyl acetate layer, wash twice with 40ml of water, add 50 grams of anhydrous sodium sulfate and 5 g of activated carbon was dried and decolorized overnight. On the second day, sodium sulfate and activated carbon were removed by filtration, concentrated until solids were precipitated, and 50 ml of petroleum ether was added to stir and crystallize. After the crystallization was c...
Embodiment 2
[0046] Embodiment 2, the preparation of compound I-1-1 of the present invention
[0047]
[0048]Dissolve 20 grams of demethylanisyltrithione (ADT) in 200 ml of dichloromethane, add 21 grams of pyridine, cool down to below -10 degrees Celsius, slowly add 27 grams of phosphorus oxychloride, and drop phosphorus oxychloride at -5 After 5 hours of reaction at ~0°C, the reaction of demethylanisyl trisulfide is complete. Add 200ml of ice water, stir for half an hour and let stand, the obtained crude compound I-1-1 sinks to the bottom of the bottle as an oily substance. The water and dichloromethane were poured off, and the residual oil was washed twice with 40 ml of 1N hydrochloric acid to remove pyridine hydrochloride. After pouring off the hydrochloric acid, the obtained product was dissolved in 100 ml of tetrahydrofuran, and dried by adding 30 g of anhydrous sodium sulfate. The sodium sulfate was removed by filtration and the tetrahydrofuran was concentrated to dryness to ob...
Embodiment 3
[0050] Embodiment 3, the preparation of compound I-1-2, I-1-3 of the present invention
[0051]
[0052]
[0053] 12 grams of compound I-1-1 was dissolved in 30 ml of absolute ethanol, and a saturated solution of sodium methoxide in methanol was slowly added dropwise with stirring at room temperature until the pH value was about 7.5, and about 2.5 grams of sodium methoxide was consumed. After the dropwise addition was completed, stirring was continued at room temperature for 30 minutes, and a reddish-brown solid was obtained by filtration and drying, that is, 11 g of crude compound I-1-2, with a yield of 80%.
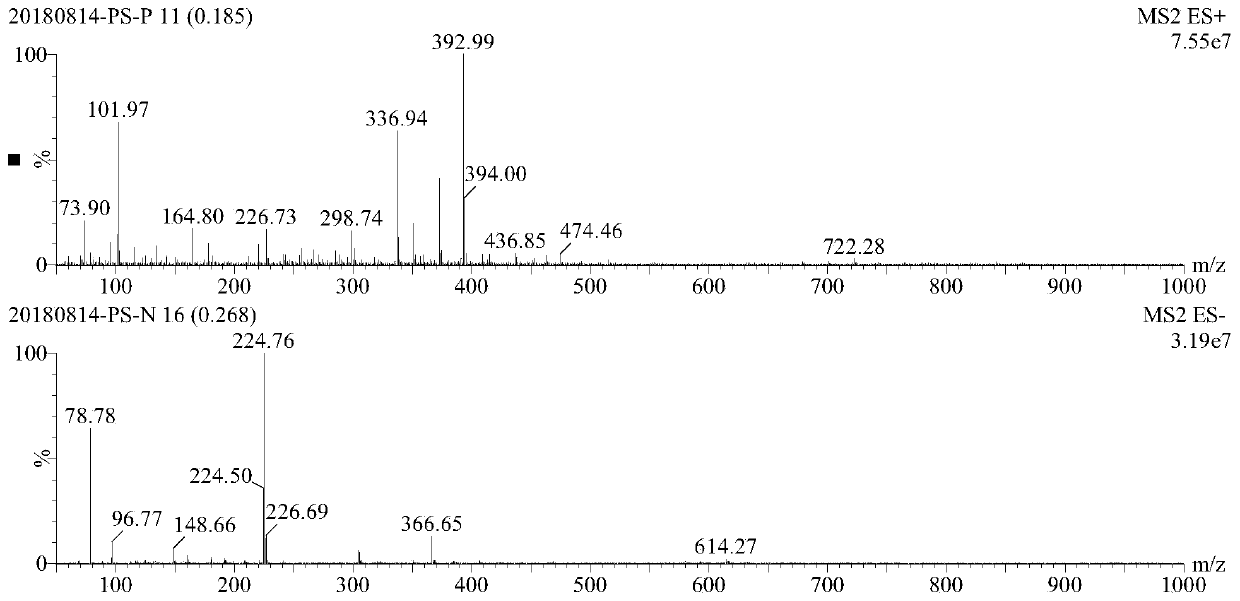
[0054] 11 grams of crude product I-1-2 was dissolved with 44 grams of pure water heated to 70 degrees Celsius, cooled naturally to room temperature, slowly added with 88 grams of absolute ethanol, the product crystallized, continued to stir for 1 hour, filtered, and dried to obtain compound I-1 -2 Light red solid 7 g, yield 63.6%. The mass spectrogram of compound...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com