A 5-azido-5-fluoro-1,3-dioxanyl-2-one derivative and its preparation method
A technology of dioxocyclyl and derivatives is applied in the field of preparation of vinyl azide compounds, and achieves the effects of moderate yield, wide substrate range and simple and efficient synthesis process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
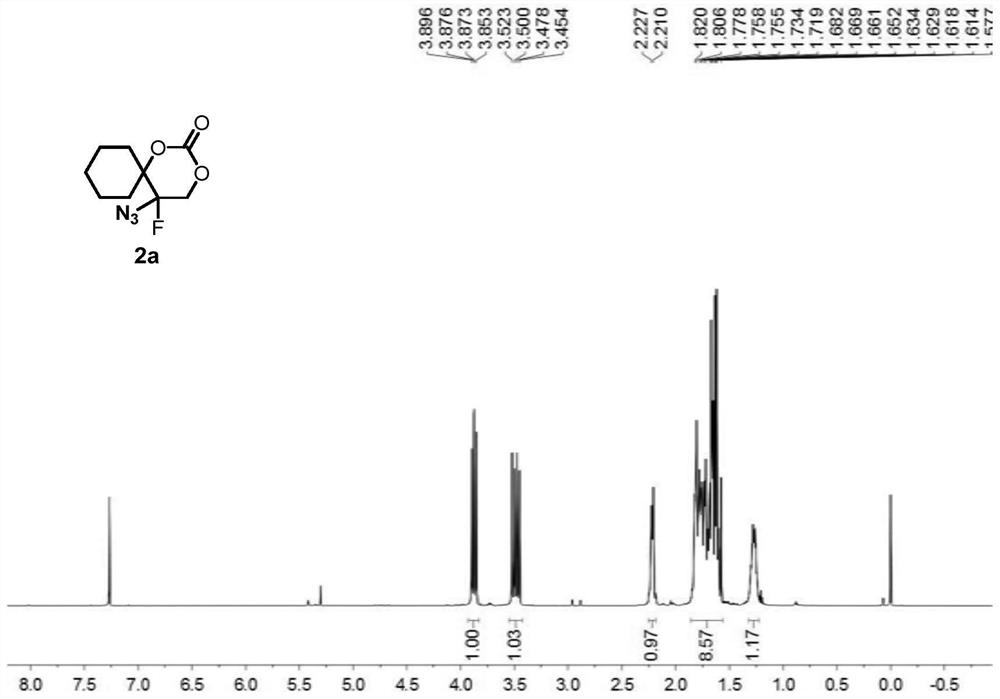
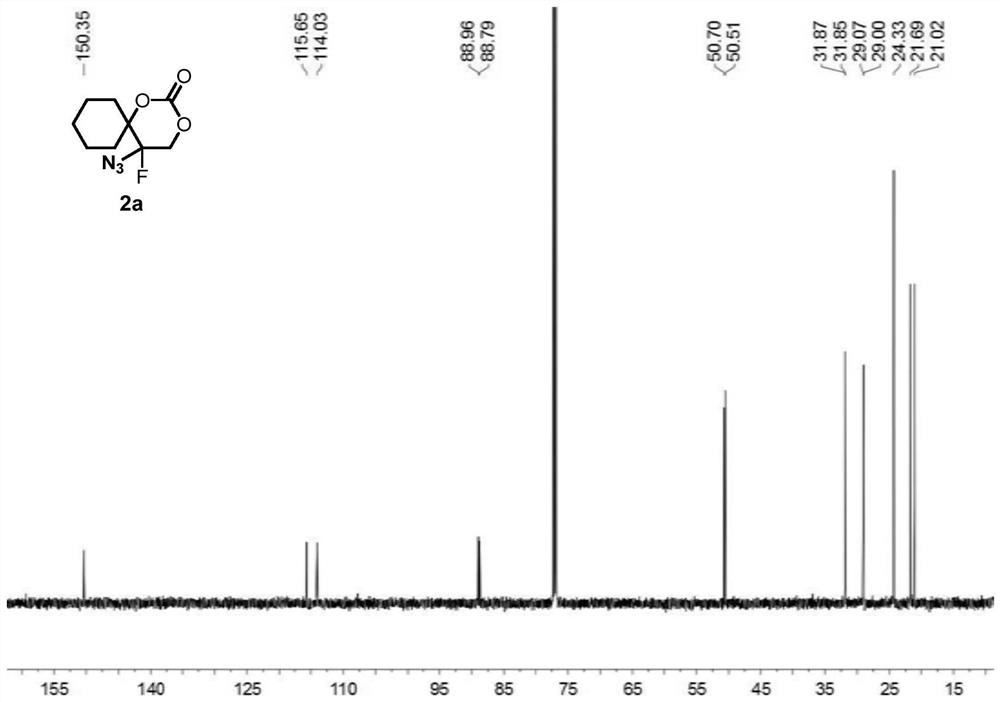
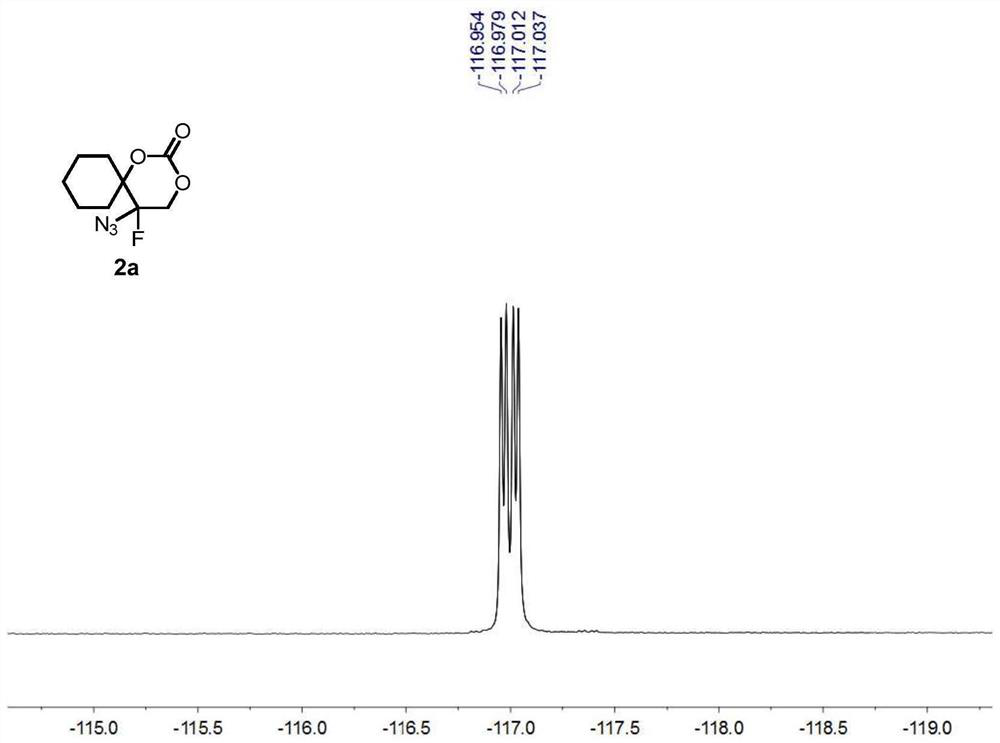
[0084] Compound 1a (i.e. 1-(1-azidovinyl)cyclohexyl tert-butyl carbonate (0.1336g, 0.5mmol), oxidant PIDA (0.3995g, 1.5mmol) was added to 2mL of solvent DCM, at 25°C, Py HF (0.4956 g, 5 mmol) was added under stirring and stirring was continued until complete consumption of vinyl azide was monitored by TLC analysis (typically 1 min). At -78° C., the suspended silica ( 15 g per 1 mmol of substrate), the resulting heterogeneous mixture was then transferred to a suspension in ethyl acetate, and the suspension was allowed to warm to room temperature. The resulting suspension was then filtered and extracted with dichloromethane (3×15 mL) for 3 times, washed 3 times with brine (3×40mL).Finally, the combined organic layer was washed with Na 2 SO 4 Drying, after removing solvent under reduced pressure, through silica gel column chromatography (eluent is V 石油醚 :V 乙酸乙酯 =10:3), the colorless oily product 2a was obtained with a yield of 96%.
Embodiment 2
[0086]Compound 1a (i.e. 1-(1-azidovinyl)cyclohexyl tert-butyl carbonate (0.1336g, 0.5mmol), oxidant PhIO (0.3301g, 1.5mmol) was added to 2mL of solvent DCM, at 25°C, Add Et with stirring 3 N. HF (0.4956 g, 5 mmol), continued stirring until complete consumption of vinyl azide was monitored by TLC analysis (typically 1 min). The silica (15 g per 1 mmol of substrate) was vigorously stirred to suspend the suspension at -78°C, then the resulting heterogeneous mixture was transferred to a suspension in ethyl acetate, which was allowed to warm to room temperature. The resulting suspension was then filtered, extracted three times with dichloromethane (3 x 15 mL) and washed once with brine (3 x 40 mL). Finally, the combined organic layers were subjected to Na 2 SO 4 Drying, after removing solvent under reduced pressure, through silica gel column chromatography (eluent is V 石油醚 :V 乙酸乙酯 =5:1), the colorless oily product 2a was obtained with a yield of 71%.
Embodiment 3
[0088] Compound 1a (i.e. 1-(1-azidoethenyl)cyclohexyl tert-butyl carbonate (0.1336g, 0.5mmol), oxidant PIFA (0.6451g, 1.5mmol) was added to 2mL of solvent DCM, at 25°C, AgF ((0.4956 g, 5 mmol) was added with stirring and stirring was continued until complete consumption of vinyl azide was monitored by TLC analysis (typically 1 min). Suspended silica was vigorously stirred at -78°C (per 1 mmol substrate 15 g)), the resulting heterogeneous mixture was then transferred to a suspension in ethyl acetate, and the suspension was allowed to warm to room temperature. The resulting suspension was then filtered and extracted with dichloromethane (3×15 mL) for 3 times, washed 3 times with brine (3×40mL).Finally, the combined organic layer was washed with Na 2 SO 4 Drying, after removing solvent under reduced pressure, through silica gel column chromatography (eluent is V 石油醚 :V 乙酸乙酯 =10:3), the colorless oily product 2a was obtained with a yield of 86%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com