Therapeutic apoptotic cells for cancer therapy
An apoptotic cell, cancer technology, applied in the field of therapeutic apoptotic cells for cancer treatment, which can solve unmet and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0407] Example 1: Generation of apoptotic cells
[0408] Purpose: To generate early apoptotic cells.
[0409]Methods: Methods for preparing early apoptotic cell populations have been well documented in International Publication No. WO 2014 / 087408 and U.S. Application Publication No. US2015 / 0275175-A1, see, for example, "Preparation of Early Apoptotic Cell Populations" and "Apoptotic Cell Populations" Generation of Apoptotic Cells" Example preceding the "Methods" section (paragraph to ) and Examples 11, 12, 13 and 14, the entire contents of which are incorporated herein.
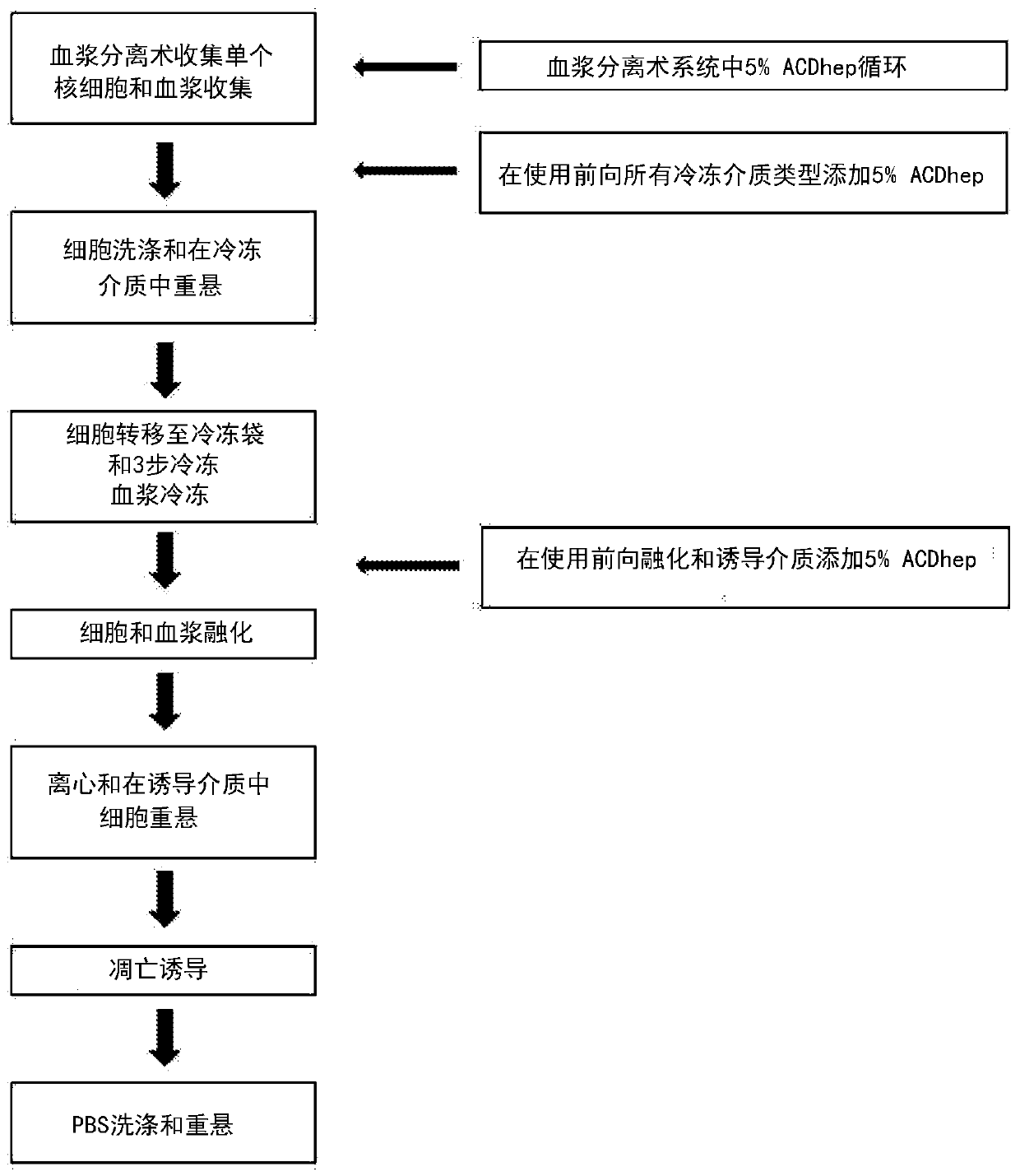
[0410] figure 1 The flow chart shown provides an overview of one embodiment of the steps used in the process of producing an early apoptotic cell population in which an anticoagulant is included in the thawing and induction of apoptosis steps. An early apoptotic cell population was prepared as described in Example 14 of International Publication No. WO2014 / 087408 and Example of U.S. Application Publication ...
Embodiment 2
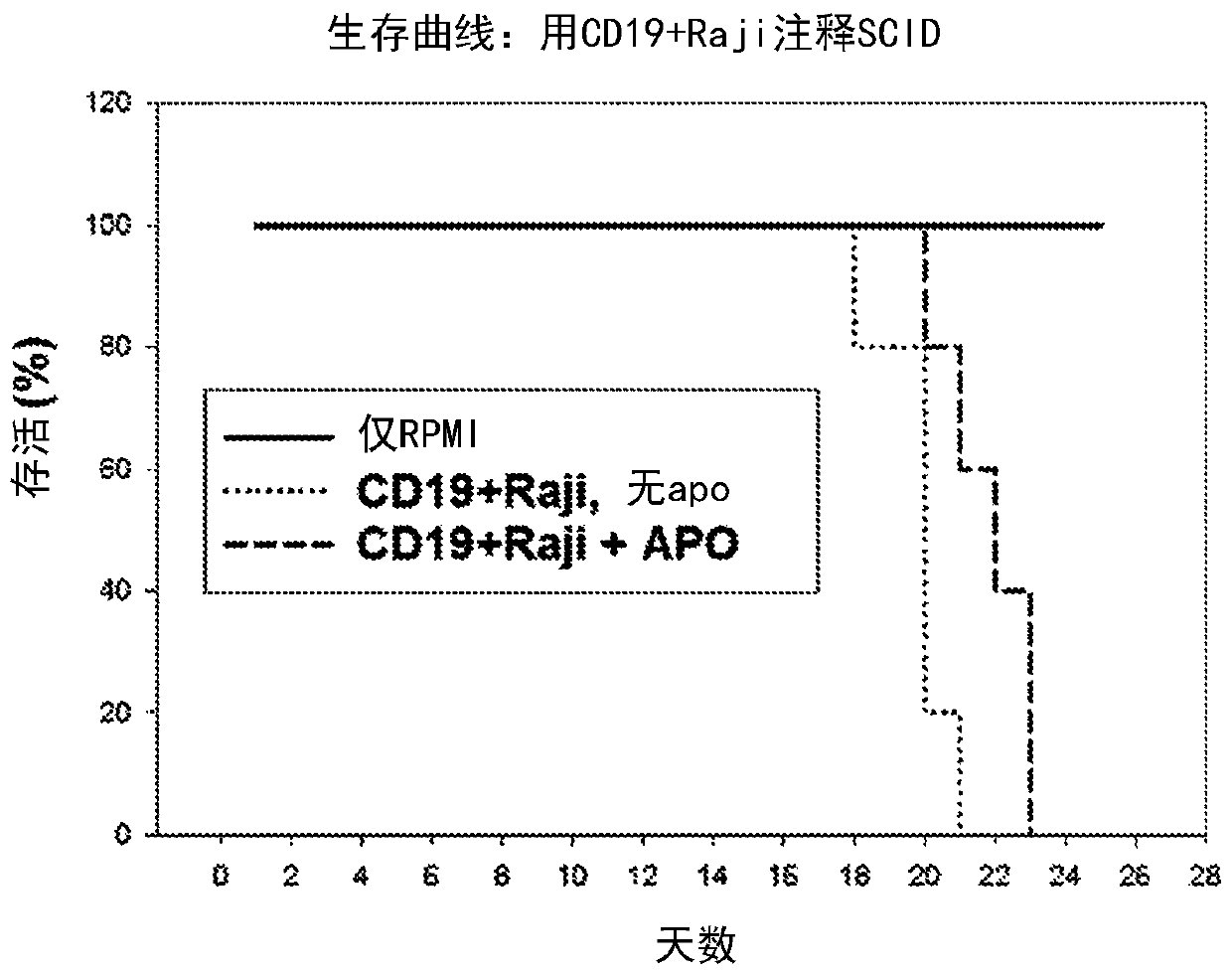
[0461] Example 2: Effects of Apoptotic Cell Treatment on Non-Solid Tumor Models
[0462] Purpose: To test the effect of apoptotic cells on widespread cancer dissemination without localized or confined non-solid tumor models to determine the efficacy of apoptotic cells on cancer survival.
[0463] method:
[0464] Raji cells
[0465] Raji cells were purchased from ECACC (catalogue number: 85011429) and routinely cultured in complete medium (RPMI-1640 supplemented with 10% H.I.FBS, 1% Glutamax, 1% penicillin / streptomycin) and maintained at 3x10 5 –3x10 6 The concentration of cells / ml.
[0466] Apoptotic cells were prepared as described in Example 1. The resulting early apoptotic cells were at least 50% Annexin V positive and less than 5% PI positive.
[0467] Non-solid (diffuse) tumor models
[0468] On day 1 of the experiment, SCID mice received 10 5 A single IV injection of Raji cells. SCID mice in the control group received a single IV injection of saline solution. (...
Embodiment 3
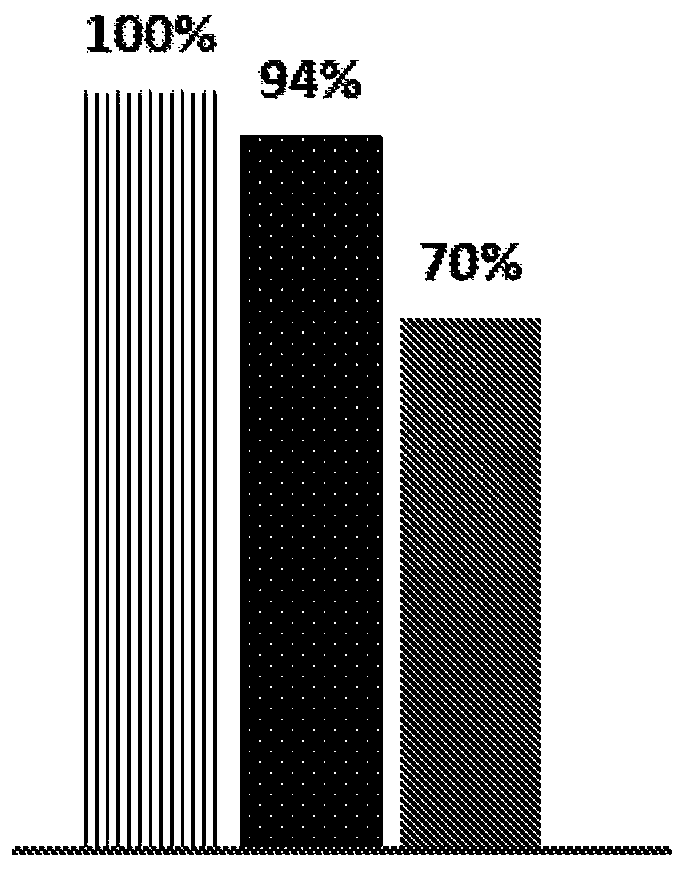
[0479] Example 3: Effect of combined treatment of apoptotic cells and anti-CD20 mAb on diffuse tumor model
[0480] Objective: To test the effect of administering a combination of early apoptotic cells and an anti-CD20 mAb in a diffuse (non-solid) tumor model, where the cancer has spread widely and is not localized or confined, to determine the efficacy of this combination therapy on patient survival.
[0481] Raji cells, apoptotic cells, non-solid (diffuse) tumor models, solid tumor models and apoptotic cells were treated as described in Example 2 above.
[0482] anti-CD20 monoclonal antibody
[0483] A commercially available anti-CD20 mAb was purchased from Roche.
[0484] Anti-CD20 mAb treatment
[0485] Mice received an IV infusion of 5 mg anti-CD20 mAb.
[0486] Combined treatment of apoptotic cells and anti-CD20 mAb
[0487] Starting on day 6 after administration of Raji cells, mice received 3 IV infusions of 30x10 6 apoptotic cells. In addition, mice received an I...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com