Compositions for use for treating cutaneous leishmaniasis
A leishmaniasis and composition technology, applied in the field of cutaneous leishmaniasis, can solve the problems of high incidence of side effects of pentavalent antimony-containing agents, hindering therapeutic application, host biological toxicity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0277] Embodiment 1: the composition of the present invention application
[0278] The composition comprising DHQ (dihydroquercetin), bisabolol, optionally alpha-tocopherol and a cosmetically and pharmaceutically acceptable carrier (oil-in-water emulsion) is given in Table 1 below.
[0279] Table 1
[0280]
[0281] The oil-in-water emulsion used as carrier in the composition of Table 1 had the following composition:
[0282] water;
[0283] sweet almond oil;
[0284] Caprylic / capric triglycerides;
[0285] Olus oil;
[0286] avocado oil;
[0287] ethanol;
[0288] cetearyl alcohol;
[0289] Dioctyl ether;
[0290] glycerin;
[0291] Propylene glycol;
[0292] Sorbitol;
[0293] Glyceryl Stearate;
[0294] Glyceryl Citrate Stearate;
[0295] Polyglyceryl-3 Methyl Glucose Distearate;
[0296] xanthan gum;
[0297] Sodium dehydroacetate;
[0298] sodium benzoate;
[0299] phenoxyethanol; and
[0300] citric acid.
Embodiment 2
[0301] Embodiment 2: Anti-Leishmania activity in vitro
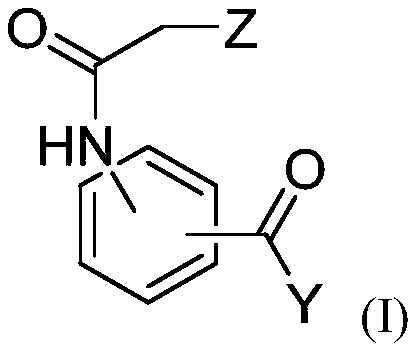
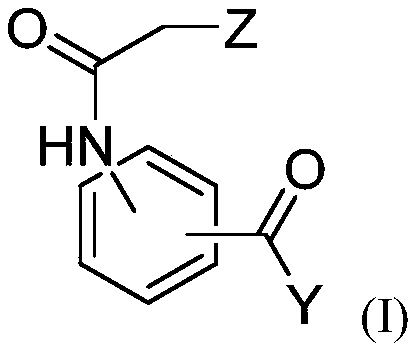
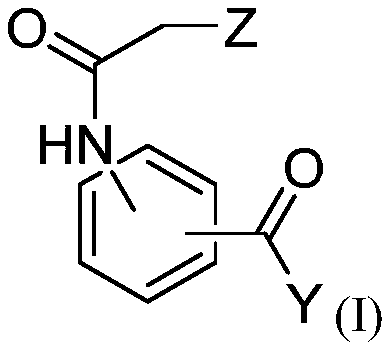
[0302] The anti-Leishmania activities of dihydroquercetin, ethyl 3-(2-chloroacetamido)benzoate and bisabolol were evaluated.
[0303] Compounds were evaluated for direct microbicidal action against Leishmania parasites in infected macrophages and neutrophils.
[0304] Bone marrow-derived macrophages (BMDM) were cultured for 7 days, collected, and infected with Leishmania major mCherry for 2 hours. Non-phagocytosed parasites were then washed away and dihydroquercetin, ethyl 3-(2-chloroacetamido)benzoate or bisabolol were added at different concentrations and incubated at different time points.
[0305] - Evaluation of parasiticidal efficacy by flow cytometry and microscopy.
[0306] - NO production was assessed by Griess reaction of the supernatant.
[0307] Neutrophils were isolated by magnetic activated cell sorting (MACS) and infected with Leishmania major mCherry at a multiplicity of infection (MOI) of 1:10.
[...
Embodiment 3
[0313] Example 3: In Vitro Toxicity Evaluation of Individual Components
[0314] with dihydroquercetin (5, 15 and 25 μM), ethyl 3-(2-chloroacetamido)benzoate (1, 2 and 4 μM) and bisabolol (0.05%, 0.1% and 0.5% w / v) Incubate for 24 hours, stain with DAPI, and assess neutrophil viability by flow cytometry.
[0315] Dihydroquercetin and bisabolol showed no cytotoxicity.
[0316] However, ethyl 3-(2-chloroacetamido)benzoate resulted in decreased neutrophil viability at the concentrations evaluated.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com