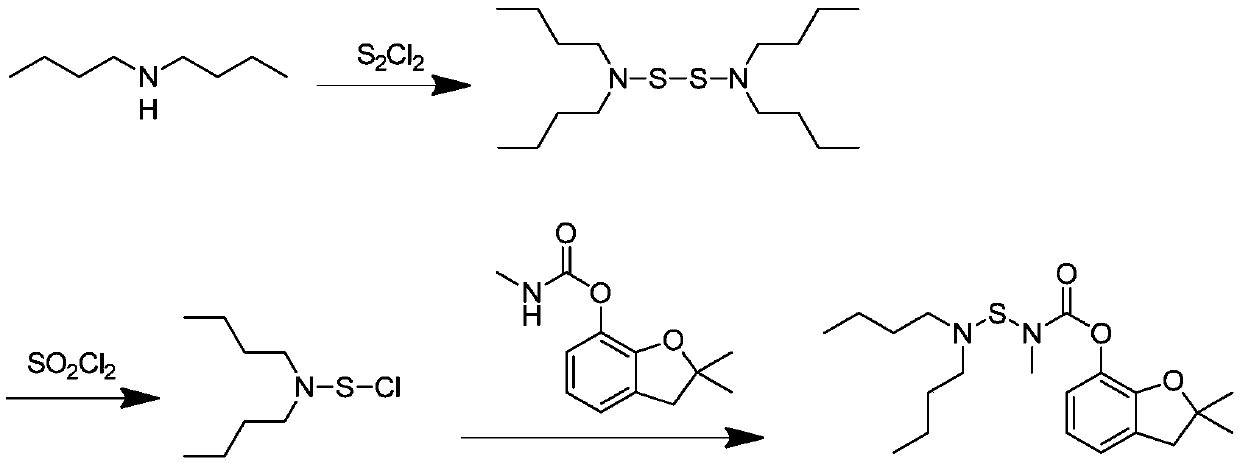
Preparation method of carbosulfan
A technology of carbosulfan and sulfide, applied in the field of pesticide preparation, can solve the problems of high recovery loss rate of co-solvent, long route, cumbersome post-treatment, etc., achieve the best target product yield and purity, and have broad prospects for popularization and application , the effect of shortening the synthesis cycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] A preparation method of Carbosulfan of the present invention, comprising the steps of:
[0042] (1) Add 110.5g (0.50mol) carbofuran and 1.0L dichloroethane into a 2L reaction flask with stirring, after the carbofuran is dissolved, add 55.6g (0.55mol) triethylamine, stir and mix Evenly, lower the temperature to 5°C, stir and add 54.6g (0.53mol) of sulfur dichloride dropwise, drop it over 1.5h, and keep it at 20°C for 2h to prepare a sulfide solution;
[0043] (2) After the insulation reaction is completed, the sulfide solution is cooled to 5°C, and 64.5g (0.5mol) of di-n-butylamine, 2.5g (0.02mol) of 4-dimethylaminopyridine, 75.8g (0.75mol) of three Ethylamine, heat preservation reaction at 25°C for 2h;
[0044] (3) After the reaction is completed, wash with water, wash with 10w.t.% hydrochloric acid, and wash the reaction solution with water, until the reaction solution is neutral, leave to stand for stratification, separate the organic phase, and precipitate under red...
Embodiment 2
[0046] A preparation method of Carbosulfan of the present invention, comprising the steps of:
[0047](1) Add 110.5g (0.50mol) carbofuran and 1.0L dichloromethane into a 2L reaction flask with stirring, after the carbofuran is dissolved, add 55.6g (0.55mol) triethylamine, stir and mix well , lower the temperature to 5°C, stir and add 54.6g (0.53mol) of sulfur dichloride dropwise, drop it over 1.5h, and keep it at 20°C for 2h to prepare a sulfide solution;
[0048] (2) After the heat preservation reaction is completed, the sulfide solution is cooled to 0°C, and 64.5g (0.5mol) of di-n-butylamine, 2.5g (0.02mol) of 4-dimethylaminopyridine, 75.8g (0.75mol) of Triethylamine, heat preservation reaction at 0°C for 2h;
[0049] (3) After the reaction is completed, wash with water, wash with 10w.t.% hydrochloric acid, and wash the reaction solution with water, until the reaction solution is neutral, leave to stand for stratification, separate the organic phase, and precipitate under r...
Embodiment 3
[0051] A preparation method of Carbosulfan of the present invention, comprising the steps of:
[0052] (1) Add 110.5g (0.50mol) carbofuran and 1.0L toluene into a 2L reaction flask with stirring, after the carbofuran is dissolved, add 55.6g (0.55mol) triethylamine, stir and mix evenly, cool down To 0°C, stir and add 54.6g (0.53mol) of sulfur dichloride dropwise, drop it over 1.5h, and keep it at 10°C for 3h to prepare a sulfide solution;
[0053] (2) After the insulation reaction is completed, the sulfide solution is cooled to 5°C, and 64.5g (0.5mol) of di-n-butylamine, 2.5g (0.02mol) of 4-dimethylaminopyridine, 75.8g (0.75mol) of three Ethylamine, heat preservation reaction at 25°C for 2h;
[0054] (3) After the reaction is completed, wash with water, wash with 10w.t.% hydrochloric acid, and wash the reaction solution with water, until the reaction solution is neutral, leave to stand for stratification, separate the organic phase, and precipitate under reduced pressure to ob...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com