Anti-bacterial composition against Th1 cell-inducing bacteria
An antibacterial composition, the technology of the composition, applied in the directions of bacteria, antibacterial drugs, drug combinations, etc., can solve the problems of complex and detailed mechanisms that are not fully clear, and achieve the effect of proliferation or activation inhibition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0252]
[0253] Before SPF mice (wild-type C57BL / 6) were forced to ingest Th1 cell-inducing bacteria, the following antibiotics were administered through drinking water for 4 days. In addition, mice not administered with these antibiotics were also prepared.
[0254] Antibiotics: ampicillin (200mg / L), tylosin (500mg / L), metronidazole (500mg / L), spectinomycin (200mg / L), vancomycin (200mg / L).
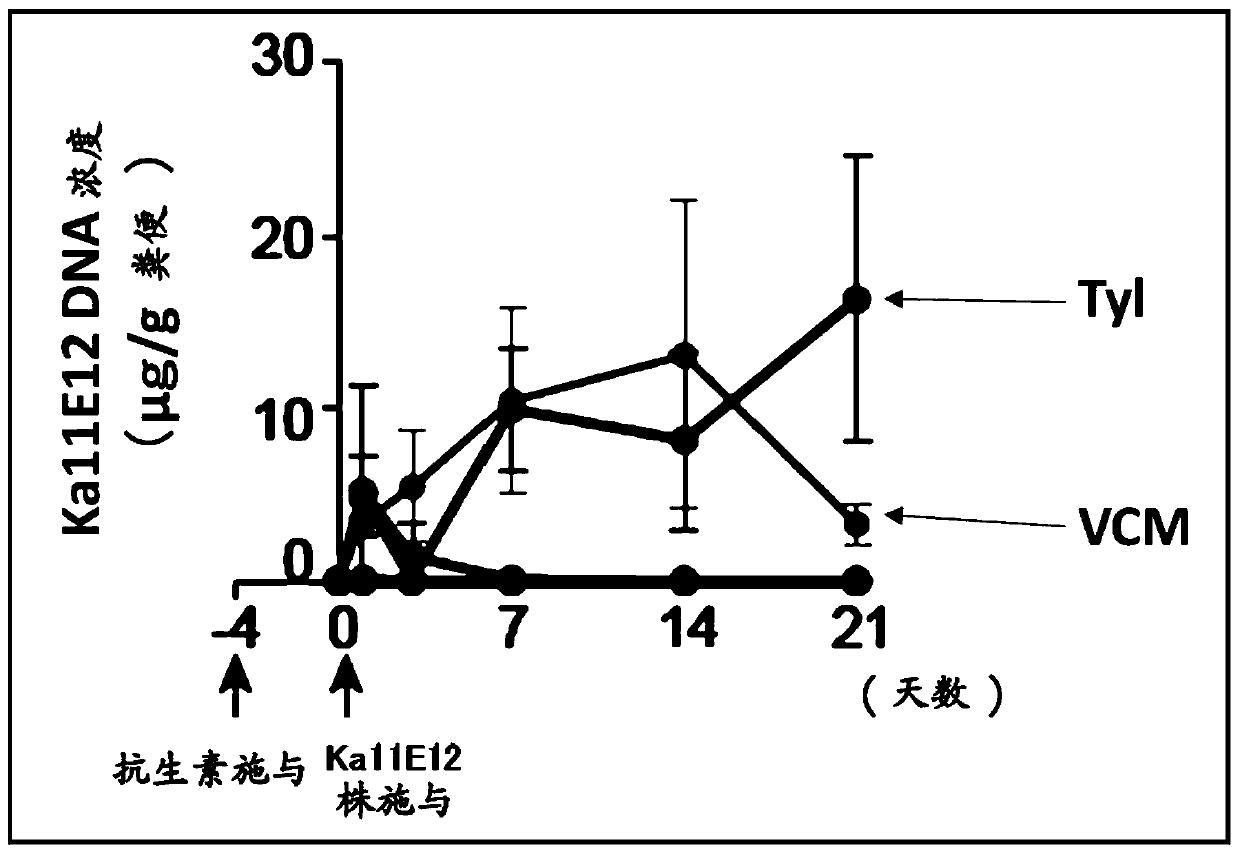
[0255] Culture Kp2H7 strain or Ka11E12 strain as a Th1 cell-inducing bacterium in LB broth to the logarithmic phase, and 1~2×10 8 CFUs were used to vaccinate mice.
[0256] After forced inoculation with Th1 cell-inducing bacteria, faeces were recovered 1, 3, 7, 14 and 21 days later, from which DNA was extracted. Then, using these DNAs as templates, qPCR was performed using the following primers specific to each bacterial strain, and the intestinal colonization amount of each bacterial strain was evaluated.
[0257] Klebsiella (ompK36-3_F: 5'-GCGACCAGACCTACATGCGT-3' [SEQ ID NO: 148], ...
Embodiment 2
[0269]
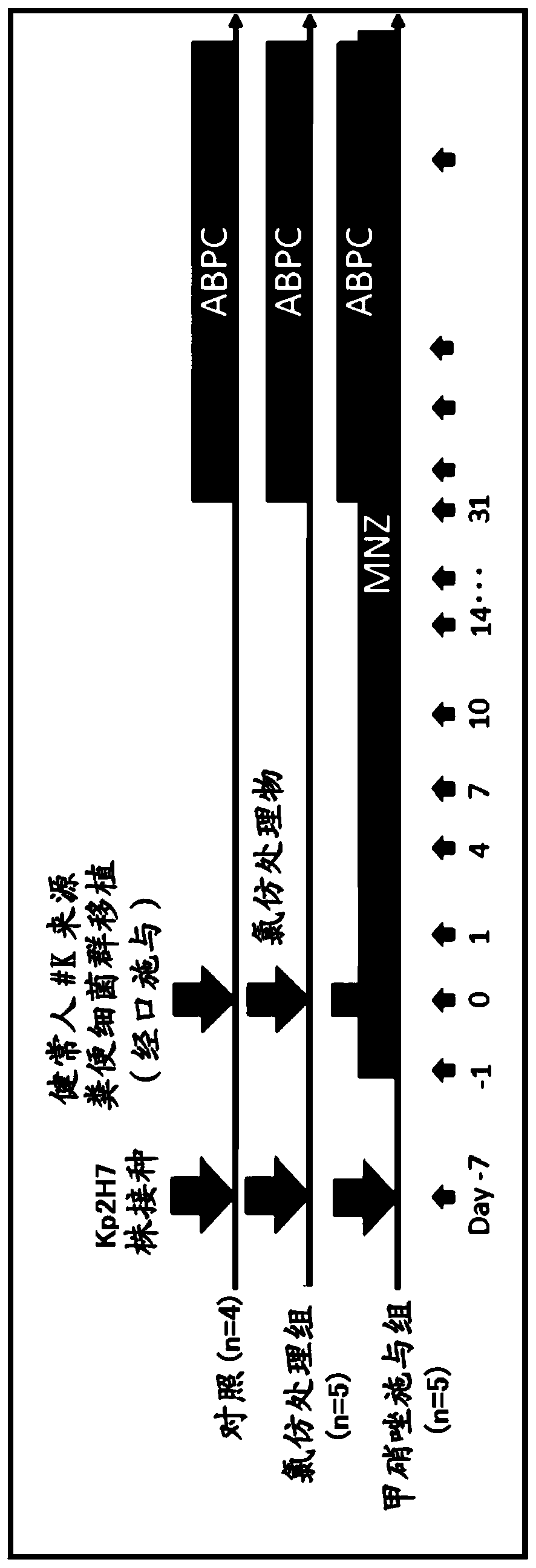
[0270] like image 3 As shown, in the same manner as in Example 1, germ-free mice were inoculated with the Kp2H7 strain. Then, one week after the inoculation, a human stool sample collected from a healthy person (#K) was orally administered. In addition, 31 to 94 days after the oral administration, administration of ampicillin was continued in the same manner as in Example 1 (hereinafter, the mice treated in this way are also referred to as "control").
[0271] In addition, germ-free mice were treated in the same manner as the control (hereinafter, also referred to as The treated mice were referred to as "chloroform-treated group").
[0272] Furthermore, except that metronidazole was administered in the same manner as in Example 1 one day before the oral administration of the human feces sample, the germ-free mice were treated in the same manner as the control (hereinafter, after such treatment, The mice were referred to as "metronidazole-administered group").
...
Embodiment 3
[0276]
[0277] Preparation of stool samples
[0278] The stool (#K stool sample, #F stool sample and #I stool sample) provided by each healthy volunteer (#K, F and I) was diluted to 5% with glycerol PBS solution (final concentration of glycerol: 20% by volume). Double the weight, filtered through a filter with a diameter of 100 μm, and stored at -80°C as a stock solution. In addition, the healthy person volunteer #K in this example is the same person as the healthy person #K in Example 2.
[0279] Production of Kp2H7 single-bacteria colonized mice
[0280] C57BL / 6N Jcl gnotobiotic mice (manufactured by Nippon CLEA Co., Ltd., 4 to 8 weeks old) were kept in a vinyl isolation box (sterile isolation box) for breeding (manufactured by Aishiem Co., Ltd.; ICM-1B) with free access to drinking water. The feeding conditions were raised for more than 1 week to carry out environmental acclimatization.
[0281] The Kp2H7 strain was cultured with Schaedler's blood medium, LB mediu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com