Application of carbinoxamine maleate in preparation of anti-influenza virus medicine
A technology for carboximin maleate and anti-influenza virus, applied in the field of medicine, can solve problems such as restricted use and not widely used, and achieve the effect of solving drug resistance and broad-spectrum antiviral activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] CAM activity in vitro Compound Example 1 in the embodiment of anti-influenza virus
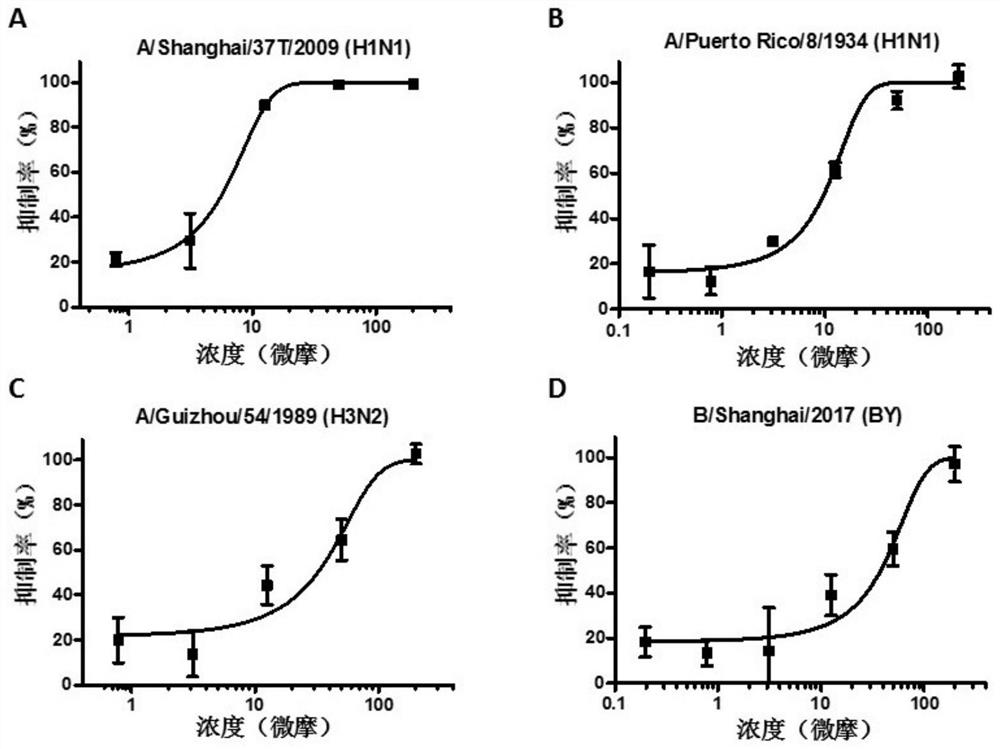
[0032] In vitro experiments The present invention relates to antiviral multiple subtypes of influenza A and B viruses, including A / Shanghai / 37T / 2009 (H1N1), A / Puerto Rico / 8 / 1934 (H1N1), A / Guizhou / 54 / 1989 (H3N2) and B / Shanghai / 2017 (BY), specifically as follows:
[0033] MDCK cells were 2 × 10 4 / Well in 96 well plates at 37 ℃, 5% CO 2Thermostat incubator to cell monolayers. Compound was added to each well and 50 l of serial dilutions of 50μl 100TCID 50 After influenza virus infected cells were mixed, incubated 8h 37 ℃. Discarded mixture was added DMEM (containing 2μg / ml TPCK) Blank 200 l of medium per well, cultured for 48h. By CCK-8 binding was determined by the antiviral activity of the compounds of the protective effect of the compound on the cell, and further calculate the median effective concentration IC 50 ;
[0034] The results indicate that the compounds can ...
Embodiment 2
[0037] Example 2 Toxicity Test compound cell embodiment of the CAM
[0038] Compound CAM using CCK-8 method Cytotoxicity; in particular as follows:
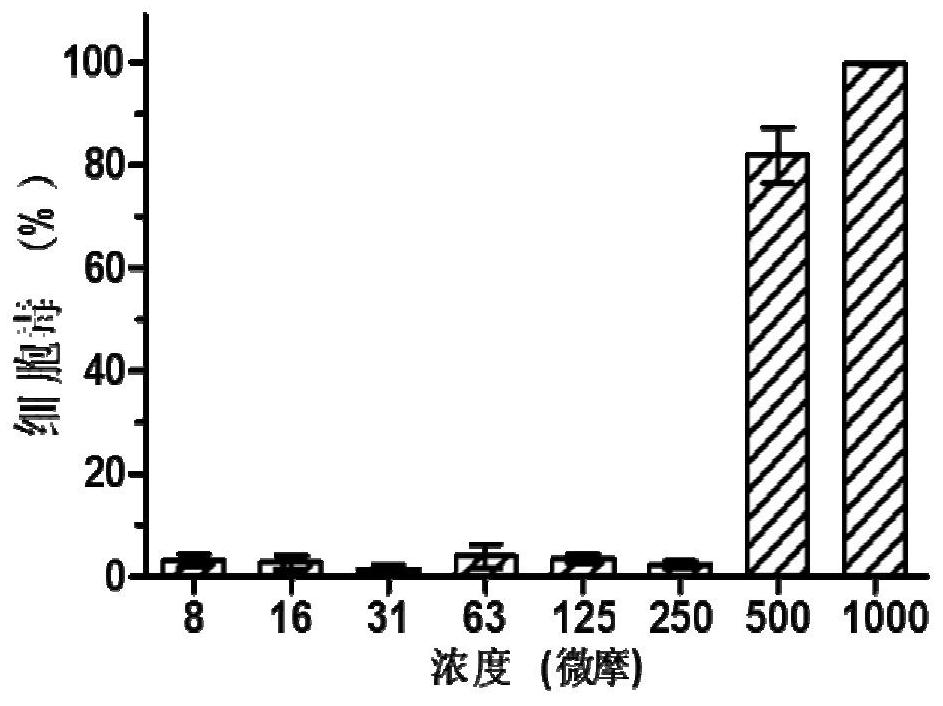
[0039] MDCK cells were 1 × 10 4 / Well in 96 well plates at 37 ℃, 5% CO 2 Thermostat incubator to cell monolayers, the serial dilutions were added to DMEM CAM 96-well plate, 100 l per well, cultured for 72h, added to each well containing 20μL CCK-8 solution is incubated for 1h 37 ℃, utilizes a versatile microplate reader (Ultra 384, Tecan, NC) detection of absorbance at 450nm, to CAM cell viability as indicators of toxicity of MDCK cells; test showed small CAM cytotoxic compounds, high security, such as figure 2 , The toxicity of the compound CAM cells is small, in the concentration range 250μM little toxicity to MDCK cells which half toxic concentration (CC 50 ) Was 240.52 ± 12.53μM ( image 3 ); The highest drug testing in the present study within the selected concentration 200μM, a safe non-toxic in the concentration range.
Embodiment 3
[0040] Example 3 Activity of compounds against influenza virus in vivo CAM embodiment
[0041] The present invention is further evaluated in vivo inhibitory activity against influenza virus CAM, 4 to 8 weeks of using C57BL / 6 female mice were infected with the H7N9 influenza virus CAM 1h intervention, the detection of infection the survival rate after 14 days, as follows:
[0042] 10LD50 using influenza A virus A / Shanghai / 4664T / 2013 (H7N9) 4 to 8 weeks of age were inoculated C57BL / 6 female mice. IH after infection, respectively, a high dose (10mg / kg / day) and low dose (1mg / kg / day) intraperitoneal injection of the CAM, continuous intervention 5 days to neuraminidase inhibitor oseltamivir phosphate (or OSEs ) (1mg / kg / day) as a positive control, PBS as a negative control group, after infection with influenza virus calculated 2,4,6,8,10,12 and 14 days survival of mice; test results show that compounds of the CAM mice infected with influenza virus has a protective e...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com