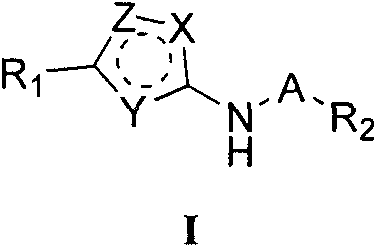
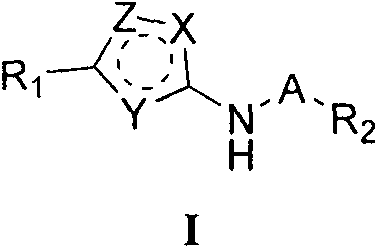
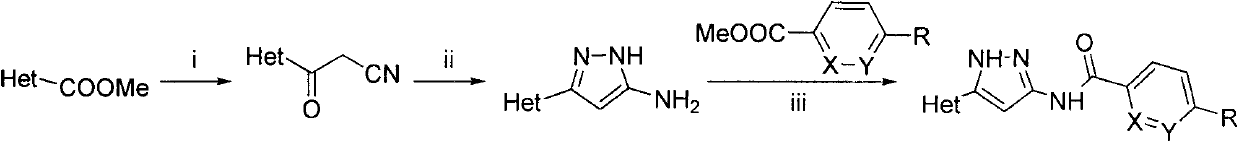
Indole substituted azole compound and applications thereof
A compound, indole technology, applied in the field of medicinal chemistry, can solve the problem of weak selectivity of kinase inhibitors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0239] N-(3-(1H-indol-2-yl)-1H-pyrazol-5-yl)-4-(4-methylpiperazin-1-yl)benzamide (1)
[0240] Into a round bottom flask were added sequentially tetrahydrofuran (12ml), acetonitrile (6mL), sodium hydride (0.17g, 7.1mmol) and methyl 1-tert-butoxyformyl-1H-indole-2-carboxylate under ice bath condition (0.688g, 2.5mmol), stirred for 0.5h and then heated to reflux for 6h. The reaction solution was poured into an ice-water mixture (50ml), and a white solid precipitated out, which was filtered and dried to obtain a crude product. After the crude product was subjected to column chromatography (PE:EA=7:1), 0.334 g of a white solid was obtained, with a yield of 47%. MS[M-H] - 283.1.
[0241] Into a 25mL round-bottomed flask, all the products from the previous step, hydrazine hydrochloride (0.231g, 2.2mmol), triethylamine (0.44g, 4.4mmol) and ethanol (10ml) were sequentially added, and the reaction was refluxed for 8h. The reaction solution was diluted with water (50ml), adjusted to ...
Embodiment 2
[0246] N-(3-(1H-indol-2-yl)-1H-pyrazol-5-yl)-4-((1-methylpiperidin-4-yl)amino)benzamide (2)
[0247] The preparation method is similar to (1), and a light yellow solid is obtained. 1 H NMR (300MHz, CDCl 3 )δ12.86(s, 1H), 11.39(s, 1H), 10.32(s, 1H), 7.71(d, 1H), 7.69-7.59(m, 3H), 7.12(t, 1H), 7.06-6.96 (m, 2H), 6.82-6.75(m, 2H), 6.07(s, 1H), 4.35(s, 1H), 3.31-3.2(m, 1H), 2.76-2.74(m, 2H), 2.18(s , 3H), 2.08-2.0(m, 2H), 1.90-1.88(m, 2H), 1.47-1.37(m, 2H). MS(m / z): [M+H] + 415.2.
Embodiment 3
[0249] N-(3-(1H-indol-6-yl)-1H-pyrazol-5-yl)-4-((1-methylpiperidin-4-yl)amino)benzamide (3)
[0250] The preparation method is similar to (1), and a light yellow solid is obtained. 1 H NMR (300MHz, CDCl 3 )δ12.86(s, 1H), 10.47(s, 1H), 10.32(s, 1H), 7.87(dd, 1H), 7.77(d, 1H), 7.66-7.58(m, 3H), 7.18(d , 1H), 6.82-6.75(m, 2H), 6.56(dd, 1H), 6.53(s, 1H), 4.35(s, 1H), 3.31-3.2(m, 1H), 2.76-2.74(m, 2H ), 2.18(s, 3H), 2.08-2.0(m, 2H), 1.90-1.88(m, 2H), 1.47-1.37(m, 2H). MS(m / z): [M+H] + 415.2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com