Amide compound containing 3-trifluoromethylpyridine, preparation method and applications thereof, and a bactericide
A technology for trifluoromethyl pyridine amide and compound, which is applied in the field of trifluoromethyl pyridine amide-containing compounds and their preparation, can solve the problems of undisclosed trifluoromethyl pyridine amide-containing compounds and the like, and achieves simple post-processing , the raw materials are cheap and easy to obtain, and the reaction conditions are mild.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
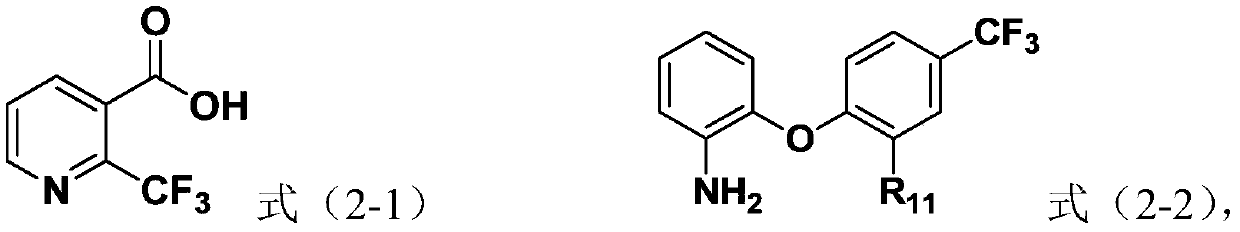
[0063] Preparation Example 1: For the preparation of the compound shown in the intermediate formula (2-2)
[0064] Add 3mmol of the compound represented by formula (2-3), 3.6mmol of 2-aminophenol and 4.5mmol of potassium carbonate to a 50mL round bottom flask, then add 20mL of N,N-dimethylformamide, and heat up to 100 ℃, TLC monitors that the reaction of raw materials is stopped after the completion of the reaction, adding 50mL of ethyl acetate, washing with 50mL of saturated brine twice, adding anhydrous sodium sulfate to dry, removing the solvent under reduced pressure and column chromatography to obtain the intermediate (2-2). The compound shown, the structure of the compound shown in formula (2-2) is as shown in table 1.
[0065] Table 1
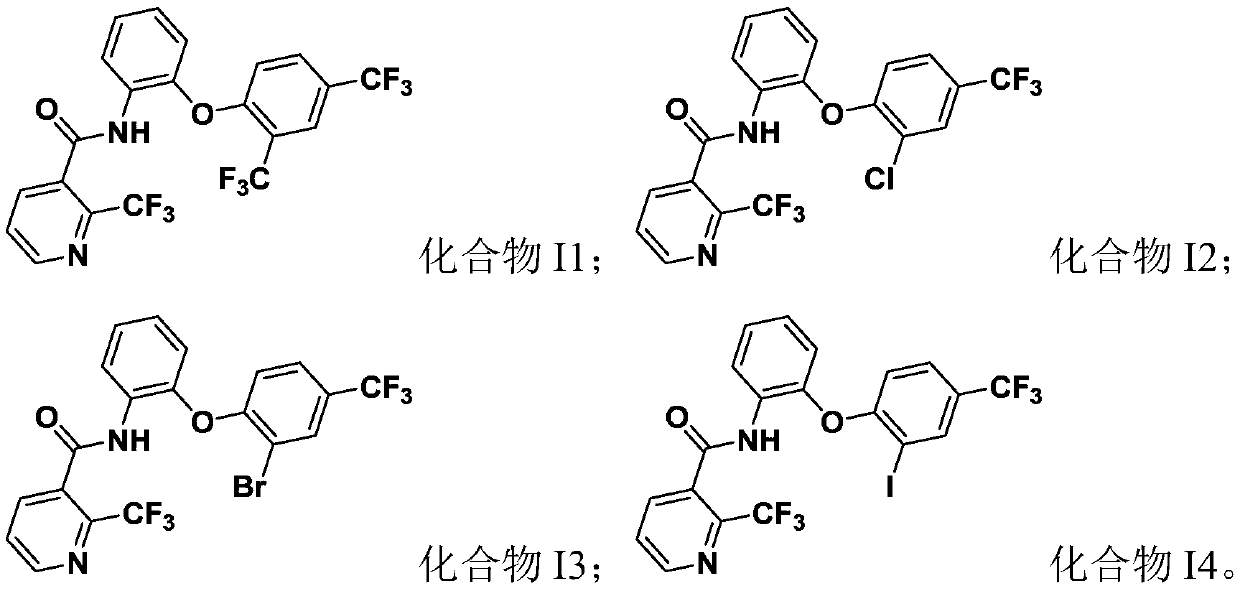
[0066] compound Substituent situation I1 R 11 for CF 3
Embodiment 1
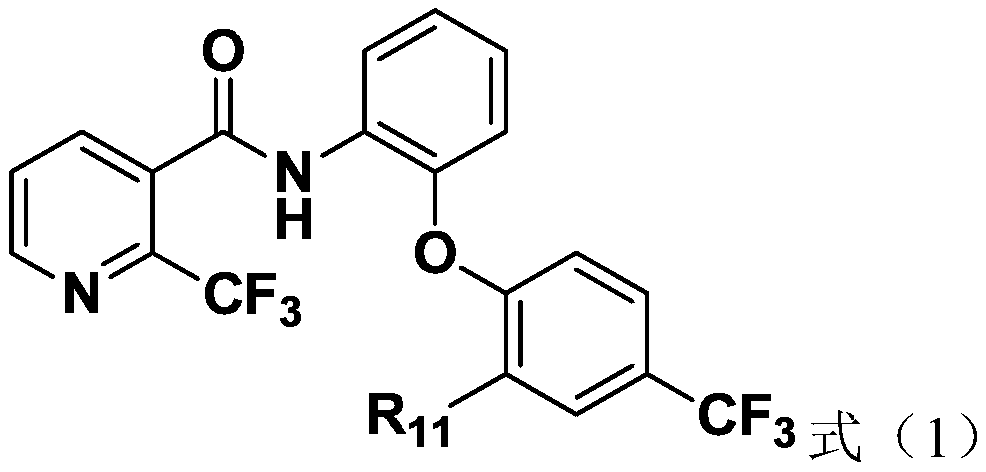
[0067] Embodiment 1: for the compound shown in preparation formula (1)
[0068] Add 2-(7-azobenzotriazole)-N,N,N',N'-tetramethyluronium hexafluorophosphate (15mmol), N,N-diiso Propylethylamine (15mmol) was dissolved in 50mL of dichloromethane, stirred evenly, then added 2-(trifluoromethyl)nicotinic acid (12mmol), and after stirring at room temperature for 2h, the formula (2- 2) The compound shown (10mmol) was added to the above solution, and TLC was used to monitor the reaction of the raw materials. After 4 hours of reaction at room temperature, the reaction was stopped. The system was washed twice with 50mL saturated brine, the organic phase was dried with sodium sulfate, and the solvent was removed under reduced pressure. Column chromatography The target compound was obtained as a yellow solid (the yield of the target product is the yield of one-step reaction).
[0069] Specifically, the structure and characterization data of the target compound are as follows:
[0070] Co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com