Pharmaceutical compositions
A composition and drug technology, applied in the field of treatment of movement disorders, can solve problems that have not been proved by experiments
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0465] A study of the properties of dihydrotetrabenazine metabolites formed after administration of tetrabenazine to human subjects
[0466] Pharmacokinetic studies were performed to determine the plasma concentrations of + / - alpha and + / - beta dihydrotetrabenazine in healthy adult male volunteers following oral administration of 25 mg tablets once and multiple times daily under fasting conditions Level. The data are summarized below.
[0467] Table 1 summarizes the pharmacokinetic data obtained after a single oral dose of tetrabenazine at a dose level of 25 mg (fasted, N=08).
[0468] Table 1
[0469]
[0470] Table 2 summarizes the pharmacokinetic data obtained after multiple oral administrations of tetrabenazine at the 25 mg dose level (fasted, N=07).
[0471] Table 2
[0472]
[0473] The data shown in Tables 1 and 2 indicate that, in humans, the major metabolites are the (-)-α-dihydrotetrabenazine isomer, which is essentially active as a VMAT2 binder, and (+)-β ...
Embodiment 2
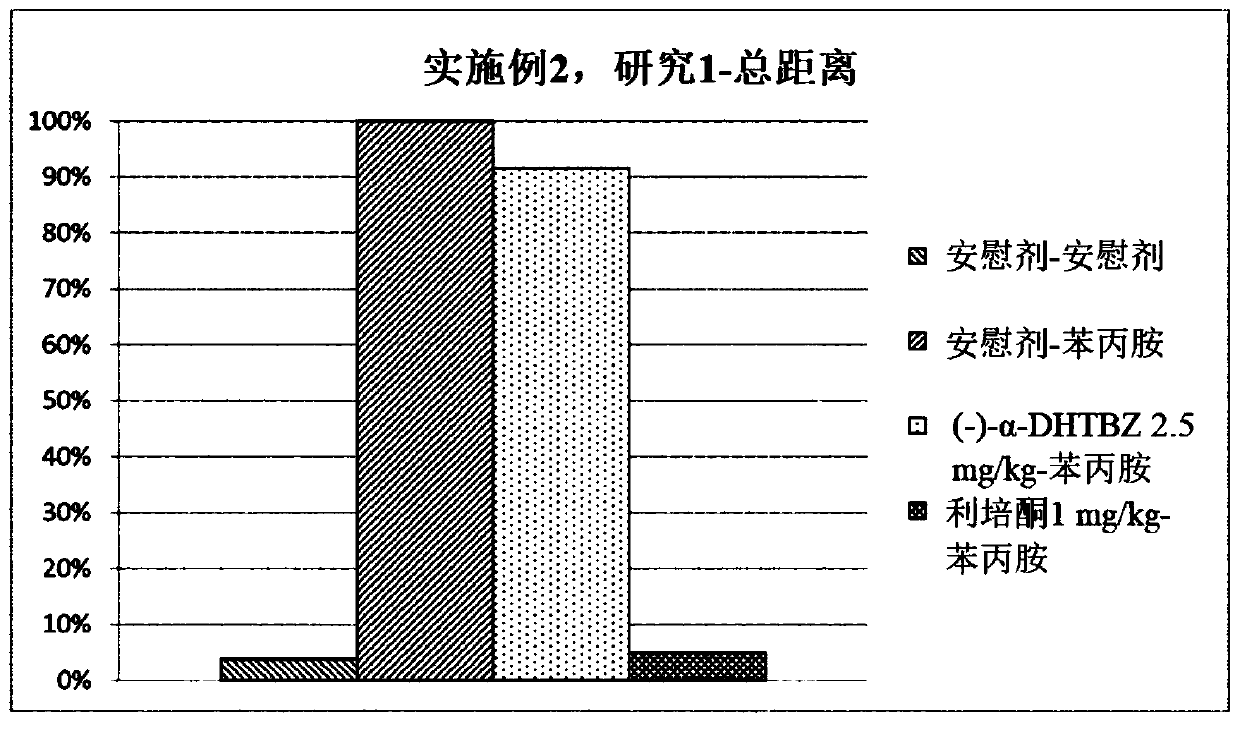
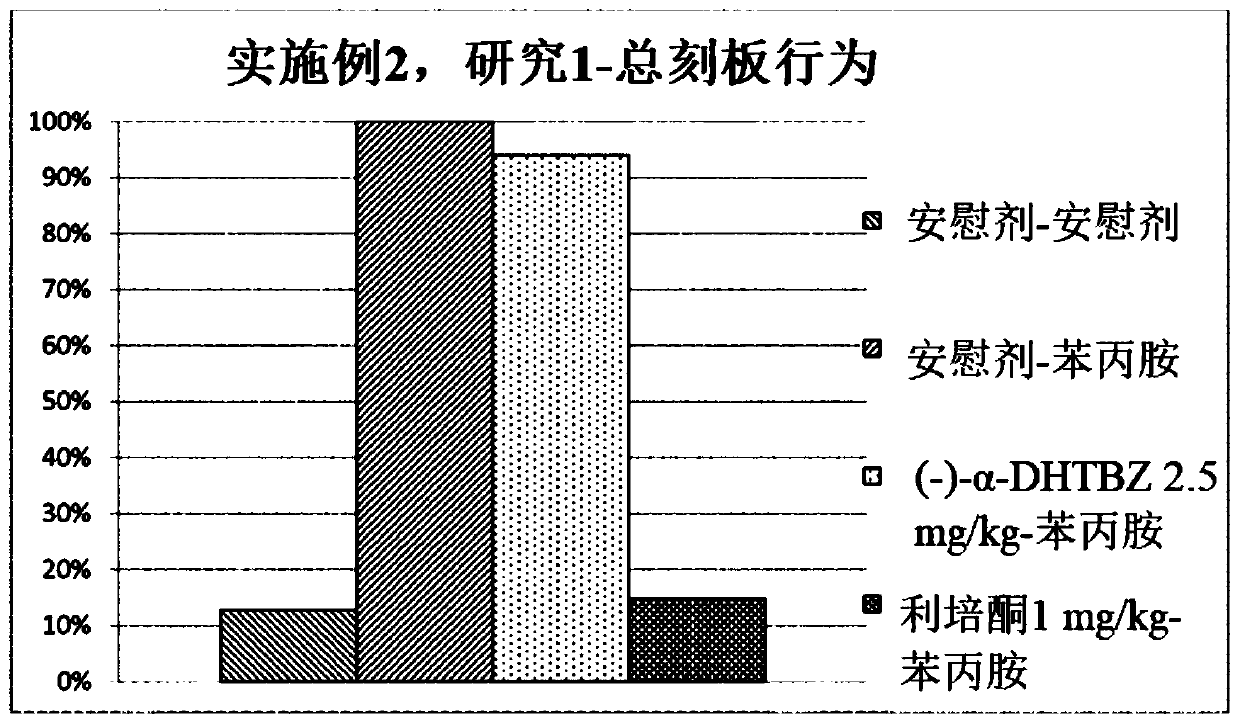
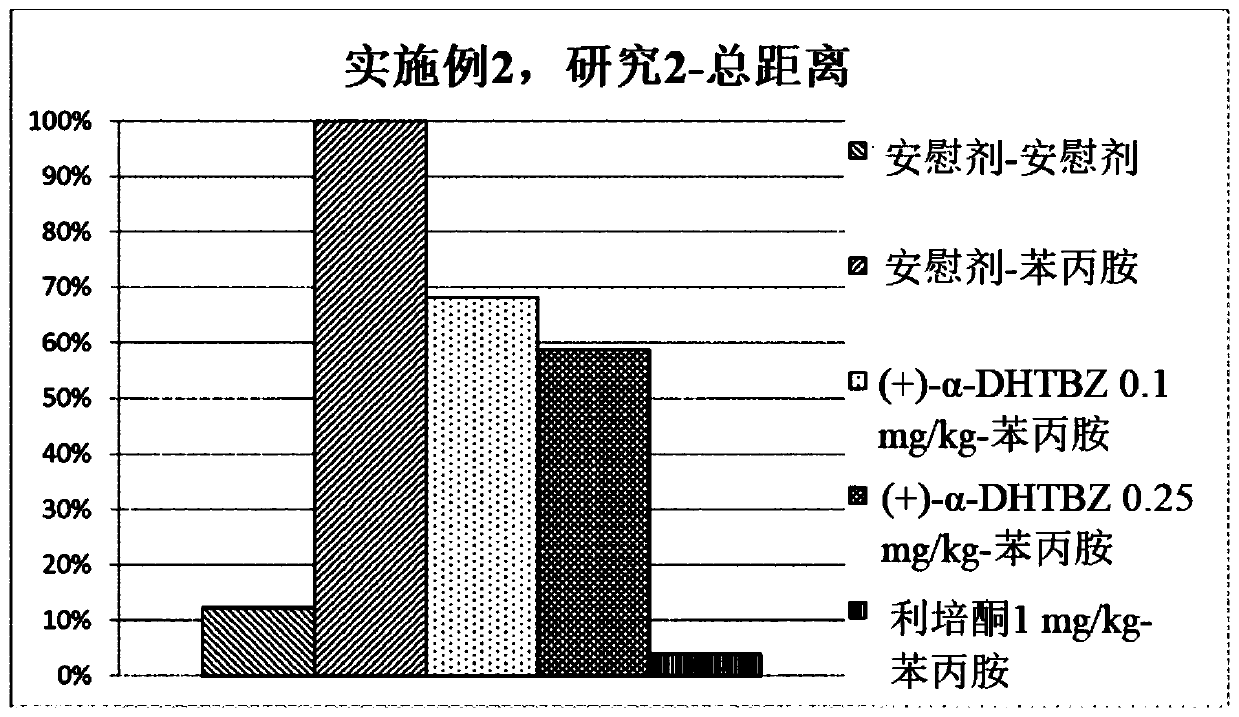
[0476] Study of the Effects of Compositions of (+)-α-Dihydrotetrabenazine and (-)-α-Dihydrotetrabenazine on Locomotor Activity and Stereotyped Behavior in Rats
[0477] The effects of a combination of (+)-α-dihydrotetrabenazine and (-)-α-dihydrotetrabenazine on locomotor activity and stereotyped behavior in rats were studied and compared with those of (+)- The effects of α-dihydrotetrabenazine and (-)-α-dihydrotetrabenazine isomers were compared.
[0478] Materials and Methods
[0479] Open Fields, Med Associates Inc.
[0480] Plastic syringe 1ml, Terumo. No.: SS-01T1
[0481] Animal gavage needle 15G, Instech Solomon, catalog number: 72-4446
[0482] Sartorius electronic balance A22101, Sartorius Weighting Technology, Germany
[0483] Needle 27G Terumo Myjector, 0.5ml, number: 8300010463
[0484] Plastic syringe 3ml, Soft-Ject, number: 8300005761
[0485] BD Microtainer K2EDTA tube number: 365975
[0486] Matrix 0.75ml, Alphanum tube, Thermo Scientific, number: 4274
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com