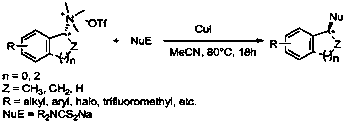
Synthetic method of dialkyl amino dithiocarbamate alkyl ester
A technology of alkyl dialkylaminodithiocarbamate and sodium phenethylaminodithiocarbamate, applied in organic chemical methods, sulfide preparation, organic chemistry, etc., can solve problems that have not been reported in the literature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
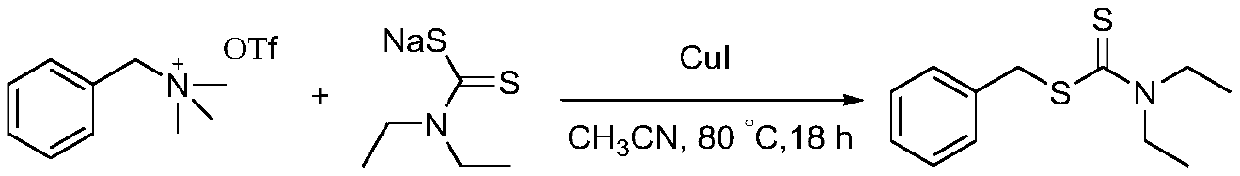
[0011] Add benzyltrimethylammonium trifluoromethanesulfonate (1.0 mmol), N,N-diethylcarbamate sodium (2.0 mmol), CuI ( 5 mol% relative to benzyltrimethylammonium trifluoromethanesulfonate), acetonitrile (3 mL), and finally the ground-mouth test tube was sealed with a rubber stopper. The test tube was placed in an 80°C oil bath and stirred for 18 hours. Then, the reaction mixture was cooled to room temperature, quenched with 15 mL of saturated NaCl solution, and extracted with ethyl acetate (3 x 20 mL). And the combined organic layers were dried over anhydrous magnesium sulfate, then adsorbed onto some silica gel under reduced pressure on a rotary evaporator. Transfer the silica gel with adsorbed sample to a silica gel column. After purification by silica gel column chromatography (petroleum ether: ethyl acetate = 20:1 as eluent), yellow oily liquid N,N-diethylcarbamate benzyl ester was obtained with a yield of 95%. The reaction equation is shown below.
[0012]
[0013]...
Embodiment 2
[0018] (o-methylbenzyl)trimethylammonium trifluoromethanesulfonate instead of benzyltrimethylammonium trifluoromethanesulfonate in Example 1 to obtain yellow oily liquid N,N-diethylaminodithioformic acid The yield of (2-methylbenzyl)ester was 95%.
[0019] 1 H NMR (400MHz, CDCl 3 )δ7.28(dd,J=7.2,1.6Hz,1H),7.16–7.03(m,3H), 4.41(s,2H),3.97(q,J=7.1Hz,2H),3.63(q,J =7.1Hz, 2H), 2.32(s, 3H), 1.19(dd, J=12.9, 5.8Hz, 6H).
[0020] 13 C NMR (101MHz, CDCl 3 )δ195.42, 137.44, 133.34, 130.48, 130.45, 127.90, 126.19, 49.26, 46.69, 40.83, 19.34, 12.43, 11.60.
[0021] MS(EI):m / z(%)=253(50)[M] + ,220(5),148(100),105(90),88(40).
Embodiment 3
[0023] (m-methylbenzyl)trimethylammonium trifluoromethanesulfonate instead of benzyltrimethylammonium trifluoromethanesulfonate in Example 1 to obtain yellow oily liquid N,N-diethylaminodithioformic acid The yield of (3-methylbenzyl)ester was 92%.
[0024] 1 H NMR (400MHz, CDCl 3 )δ7.24–7.06(m,3H),7.05–6.90(m,1H),4.42(s,2H),3.97(q,J=7.0Hz,2H),3.65(q,J=7.2Hz,2H ),2.26(s,3H),1.20(q,J=5.9, 5.2Hz,6H).
[0025] 13 C NMR (101MHz, CDCl 3 )δ195.39, 138.29, 135.76, 130.15, 128.52, 128.30, 126.48, 49.43, 46.74, 42.33, 21.38, 12.51, 11.63.
[0026] MS(EI):m / z(%)=253(60)[M] + ,207(10),148(100),105(70),88(55).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com