Method for synthesizing tulobuterol
A technique for synthesizing appropriate compounds, which is applied in the field of drug synthesis, can solve problems such as high reaction temperature, unfavorable industrial production, and low yield, and achieve the effect of simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
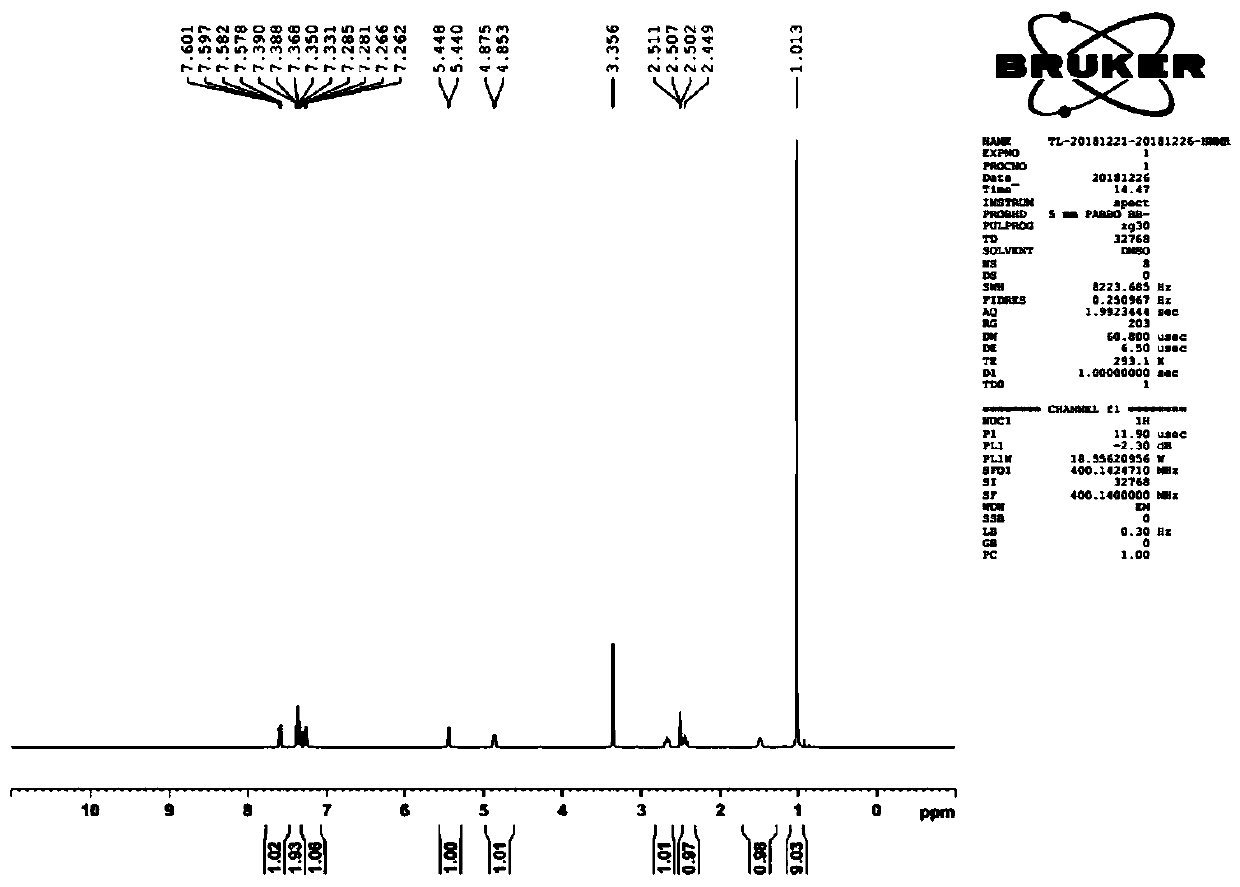
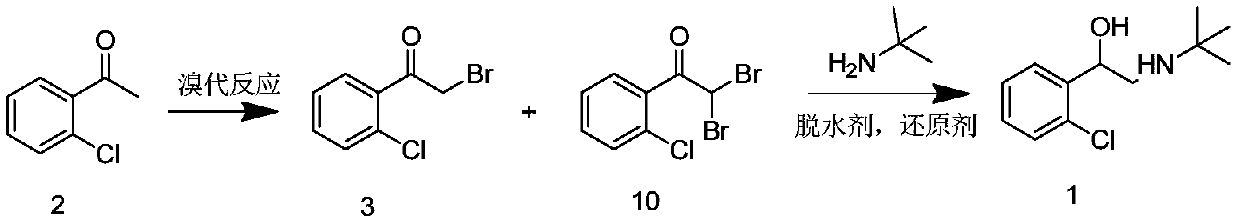
[0034] Put 100.0 g of the mixture of compound 3 and compound 10 into the three-necked flask (converted to 0.40 mol of compound 2), add 500 ml of ethanol, add 227.2 g of anhydrous sodium sulfate, add 116.8 g of tert-butylamine, and react at 30 ° C for 4 h under nitrogen protection. Cool down to -10°C, add 9.1g of sodium borohydride in portions, control the temperature at -10°C-10°C, add sodium borohydride, react at 10°C for half an hour, heat up, reflux for 6 hours, the reaction is over, concentrate the reaction solution to dryness , then add 300ml of dichloromethane and 300ml of 5% sodium hydroxide solution, extract and layer, take the dichloromethane layer, add 300ml of 4% hydrogen chloride solution, extract and separate, take the water layer, add 300ml of dichloromethane and 300ml of 5% hydroxide to the water layer Sodium solution, extraction and separation, the dichloromethane layer was extracted once with 150 ml of purified water, and concentrated to obtain 70.8 g of tulobu...
Embodiment 2
[0036] Put 100.0 g of the mixture of compound 3 and compound 10 into the three-necked flask (converted to 0.40 mol of compound 2), add 250 ml of ethanol, add 113.6 g of anhydrous sodium sulfate, add 58.4 g of tert-butylamine, and react at 30 ° C for 4 h under nitrogen protection. Cool down to -10°C, add 6.1g of sodium borohydride in portions, control the temperature at -10°C-10°C, add sodium borohydride, react at -10°C for half an hour, heat up, reflux for 8 hours, and complete the reaction, according to Example 1 After the post-processing method, 67.9 g of tulobuterol was obtained with a total molar yield of 75%.
Embodiment 3
[0038] Put 100.0 g of the mixture of compound 3 and compound 10 into the three-necked flask (converted to 0.40 mol of compound 2), add 300 ml of ethanol, add 170.4 g of anhydrous sodium sulfate, add 87.6 g of tert-butylamine, and react at 30 ° C for 3 h under nitrogen protection. Cool down to -10°C, add 7.6g of sodium borohydride in portions, control the temperature at -10°C-10°C, add sodium borohydride, react at 0°C for half an hour, heat up, reflux for 6 hours, and complete the reaction, according to the method in Example 1 After post-processing, 68.5 g of tulobuterol was obtained with a total molar yield of 75%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com