Treatment of hematological malignancy with small molecule nf-kb inhibitors
A technology for multiple myeloma and lymphoma, applied in the field of the treatment of malignant hematological tumors, which can solve the problems of different biological effects and toxicity characteristics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0086] Example 1: General Synthetic Scheme
[0087] The NF-κB inhibitors of the invention may be synthesized by any suitable method known in the art or as further described below.
[0088]
[0089] Scenario 1.2
[0090]
[0091] Scenario 1.3
[0092]
[0093] Scenario 1.4
[0094]
[0095] Scenario 1.5
[0096]
[0097] Scenario 2.1
[0098]
[0099] Scenario 2.2
[0100]
[0101] Scenario 2.3
[0102]
Embodiment 2
[0103] Embodiment 2: specific compound synthetic scheme
[0104] Preparation of IT-848
[0105]
[0106] Step 1: Preparation of 2-chloro-3-(hexyloxy)phenol
[0107] A three-neck round bottom flash (reactor) with a capacity of 500 mL was equipped with a magnetic stirrer, reflux condenser and thermometer. 18-crown-6 (3.17g, 12mmol), NaI (26.98g, 180mmol), K 2 CO 3 (33.17g, 240mmol), 2-chlororesorcinol (29.91g, 200mmol), acetone (116mL) and 1-bromohexane (29.17g, 180mmol) were added to the reactor in this order. The mixture was heated with an oil bath. The oil bath temperature was set at 72°C. The temperature in the reactor reached 60°C in a stable reaction state. The mixture becomes thicker and thicker. 5 hours after the start of the reaction, 29 mL of acetone was further added to the mixture. After 9 hours the reaction was stopped and cooled to room temperature. Once the reaction mixture reached room temperature, 150 mL of deionized water and 150 mL of ethyl acetate...
Embodiment 4
[0127] Example 4: Mice, cell lines and primary cells
[0128] Cell Lines and Primary Cells
[0129] Human peripheral blood mononuclear cells (PBMC) were purified from donor blood according to accepted protocols. Mouse splenocytes are purified from mouse spleen using methods known to those skilled in the art.
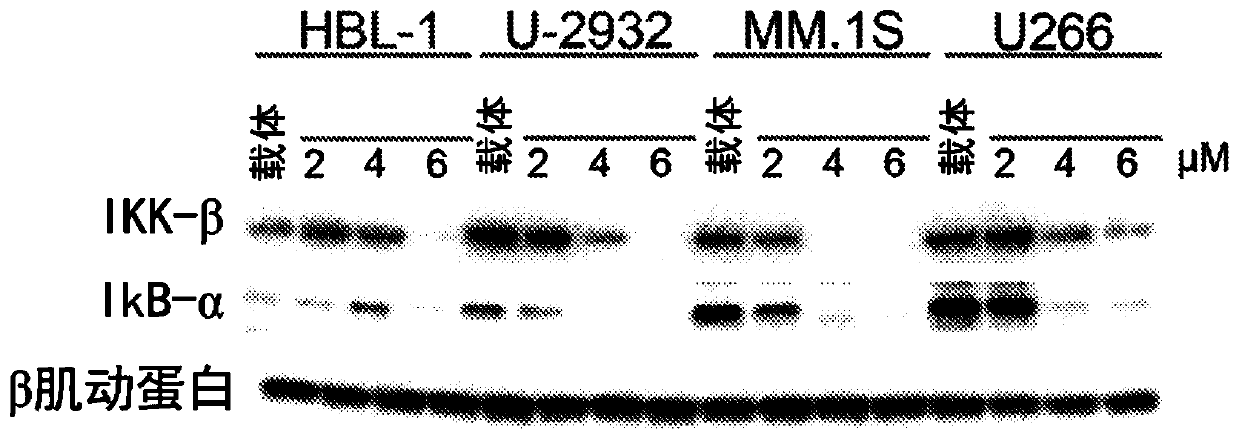
[0130] Human DLBCL-derived cell lines SU-DHL4 and U2932 were obtained from the German Collection of Microorganisms and Cell Cultures, Department of Human and Animal Cell Cultures (Braunschweig, Germany); MM.1S and U266 cells were obtained from ATCC; HBL1 cells were kindly provided by Dr. R.E. Davis (Houston, TX) and certified by the MD Anderson Characterized Cell Lines Core Facility . MM.1S cells were retrovirally transduced to express a fusion protein consisting of enhanced green fluorescent protein and firefly luciferase. Cell lines were grown in RPMI1640 medium supplemented with 10-20% fetal bovine serum, 1% L-glutamine, and penicillin-streptomycin in 5% CO 2 cul...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com