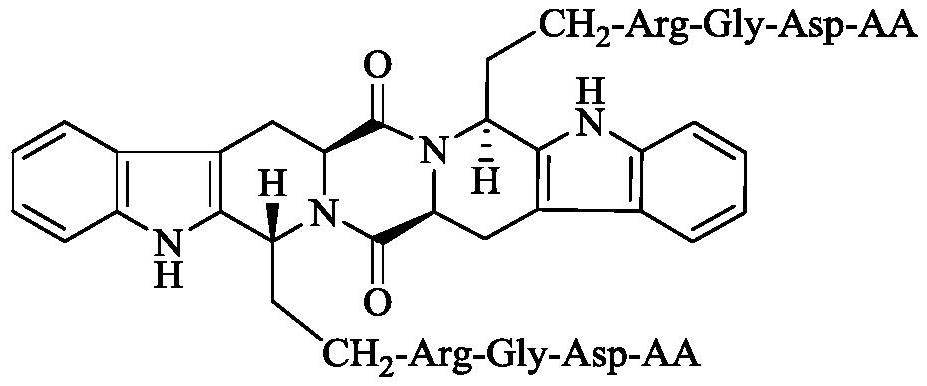
RGD tetrapeptide modified s,r-heptacyclic aldehyde, its synthesis, activity and application
A reaction, 4-b technology, application in the preparation of P-selectin antagonists, application in the preparation of GPIIb/IIIa antagonists, inhibition of in vivo P-selectin expression activity, anti-arterial thrombosis activity, S, R-heptacyclic aldehyde-Arg-Gly-Asp-AA field, can solve problems such as reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
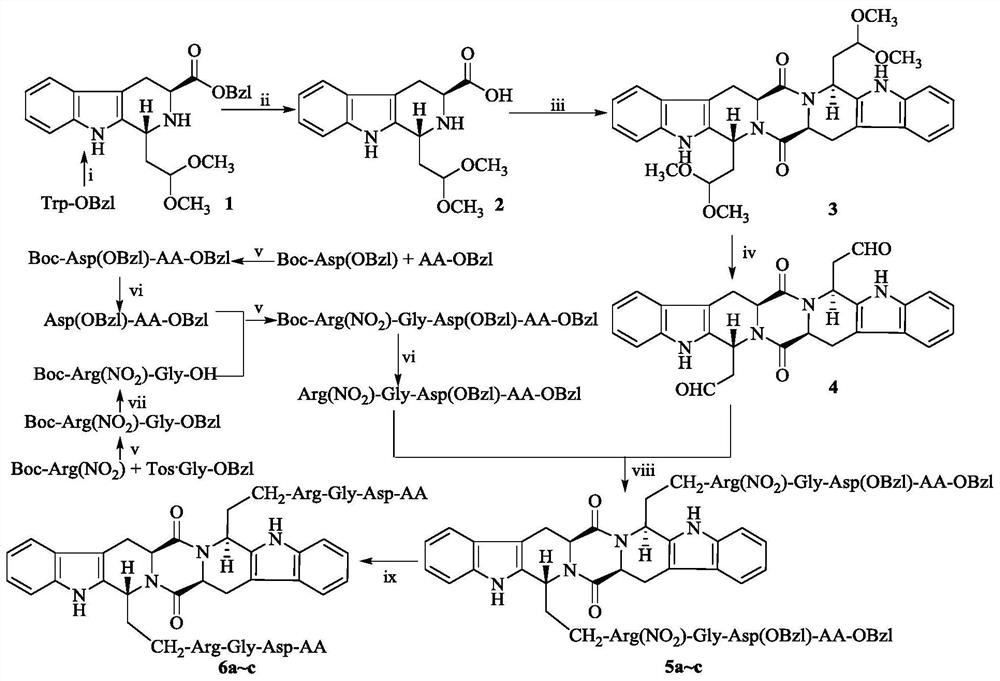
[0026]Example 1 Preparation of 1-(2,2-dimethoxyethyl)-2,3,4,9-tetrahydro-β-carboline-3-carboxylic acid benzyl ester (1)
[0027] Under ice-bath conditions, add 5 mL of 1,1,3,3-tetramethoxypropane and 5 mL of trifluoroacetic acid to 150 mL of dichloromethane in sequence, and activate in an ice-water bath for 40 min. Then 5.00 g (17.00 mmol) benzyl L-tryptophan was added. The solution was reddish brown. The reaction mixture was first stirred under ice bath for 1 h, and then stirred at room temperature for 12 h. The reaction solution was extracted and washed 6 times with saturated aqueous sodium bicarbonate solution, and a large number of bubbles would be generated. Extract and wash with saturated sodium chloride aqueous solution 3 times. The dichloromethane layer was collected and dried by adding anhydrous sodium sulfate for 2 hours. After filtration under normal pressure, the filtrate was concentrated under reduced pressure and then purified by silica gel column chromatogra...
Embodiment 2
[0028] Example 2 Preparation of 1-(2,2-dimethoxyethyl)-2,3,4,9-tetrahydro-β-carboline-3-carboxylic acid (2)
[0029] Add 540 mg of Pd / C to 5.39 g (13.68 mmol) of compound 1 and 100 mL of methanol solution, feed H 2 , Stir the reaction at room temperature for 4h. Pd / C was filtered off, and the filtrate was concentrated under reduced pressure to obtain 3.34 g (11.00 mmol) of the title compound as a yellow oil, with a yield of 80%. ESI-MS(m / e): 303[M-H] - .
Embodiment 3
[0030] Example 3 Preparation of (2S,5S)-tetrahydropyrazine[1,2:1,6]bis{(1S,1R)-[1-dimethoxyethyl-2-yl]-2,3, 4,9-tetrahydro-1H-pyridino[3,4-b]indole}-1,4-dione (3)
[0031] Suspend 3.34g (11.00mmol) of compound 2 in 60mL of N,N-dimethylformamide, add 1.49g (11.00mmol) of N-hydroxybenzotriazole and 2.72g of (13.20 mmol) dicyclohexylcarbodiimide, and then adjust the pH of the reaction solution to 8-9 with N-methylmorpholine. Reaction at room temperature for 8 hours. The reaction solution was filtered under reduced pressure, and the filtrate was concentrated under reduced pressure. Dissolve it with 100 mL of ethyl acetate, and a white solid is precipitated or insoluble. Filter under reduced pressure and collect the filtrate. The obtained ethyl acetate solution was washed successively with saturated aqueous sodium bicarbonate solution 3 times, saturated aqueous sodium chloride solution 3 times, 5% potassium bisulfate aqueous solution 3 times, saturated sodium chloride aqueous s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com