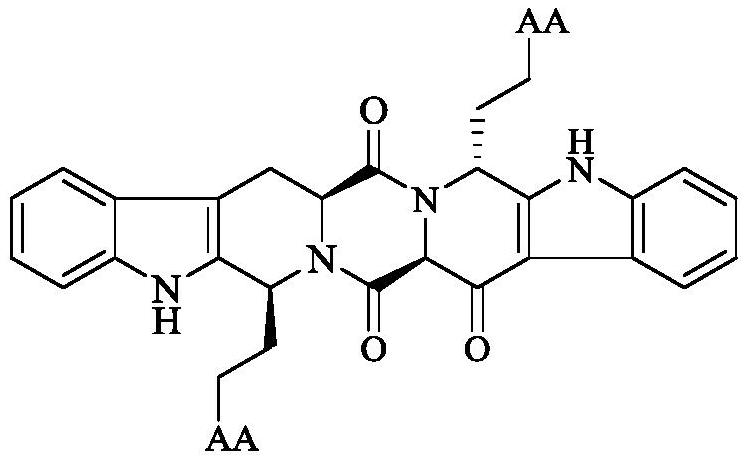
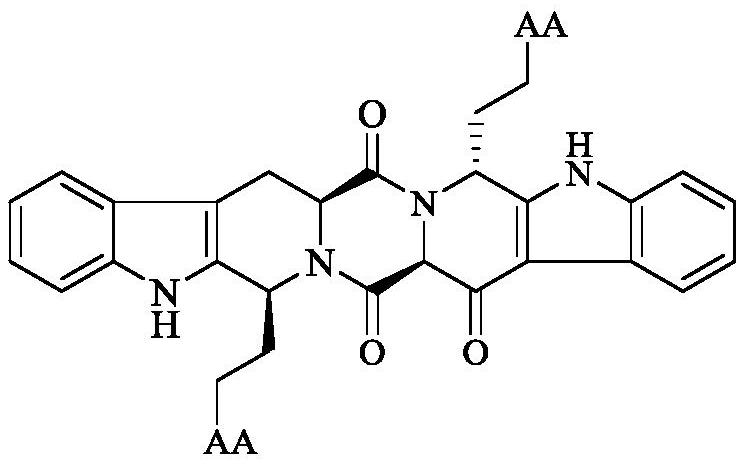
Amino Acid Modified s,r-Heptacyclic Aldehyde, Its Synthesis, Activity and Application
A technology based on residues and ethyl groups, which can be used in drug combinations, blood diseases, extracellular fluid diseases, etc., and can solve problems such as increasing adverse reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
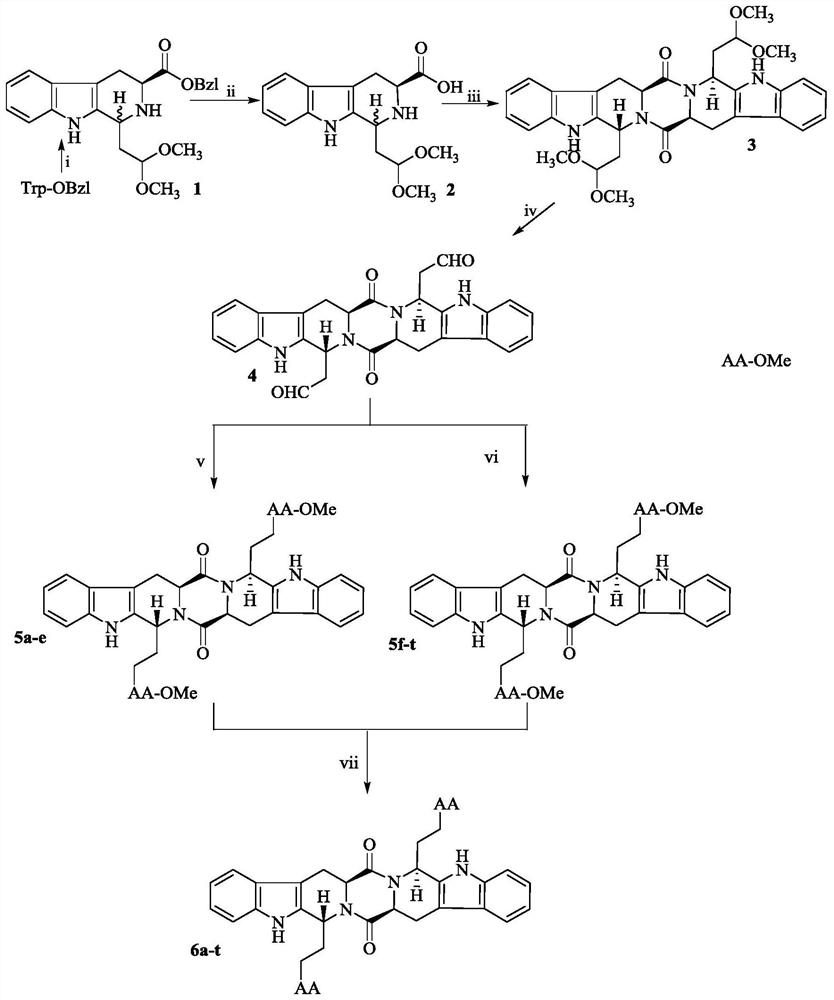
[0017] Example 1 Preparation of 1-(2,2-dimethoxyethyl)-2,3,4,9-tetrahydro-β-carboline-3-carboxylic acid benzyl ester (1)
[0018] Under stirring in an ice bath, 150mL CH was added to 5g (17.0mmol) L-Trp-OBzl 2 Cl 2, 5mL 1,1,3,3-tetramethoxypropane, 5mL trifluoroacetic acid. After reacting for 14h, point TLC plate to monitor the disappearance of the raw material point, and a new point is produced (CH 2 Cl 2 :CH 3 OH=30:1), the reaction was terminated. The reaction solution was washed with saturated NaHCO 3 Extract and wash 3 times, extract and wash 3 times with saturated NaCl, combine CH 2 Cl 2 layer, anhydrous NaSO 4 Dry for 2h, filter under reduced pressure, and purify the filtrate with silica gel column chromatography after concentrating under reduced pressure (CH 2 Cl 2 :CH 3 OH=100:1), to obtain 5.87 g (87%) of the title compound as a brown-red oil. ESI-MS(m / e):393[M+H] - .
Embodiment 2
[0019] Example 2 Preparation of 1-(2,2-dimethoxyethyl)-2,3,4,9-tetrahydro-β-carboline-3-carboxylic acid (2)
[0020] In 3.96g (10.0mmol) of 1, add 150mL CH 3 Dissolve OH, add 400mg Pd / C, fill with hydrogen and stir the reaction at room temperature. After 18 hours of reaction, point the TLC plate to monitor the disappearance of the raw material point, and a new point is generated (CH 2 Cl 2 :CH 3 OH=30:1), the reaction was terminated. After vacuum filtration, the filtrate was concentrated under reduced pressure to obtain 2.726 g (8.9 mmol) of a yellow solid, with a yield of 89%. ESI-MS(m / e):303[M-H] - .
Embodiment 3
[0021] Example 3 Preparation of (2S,5S)-tetrahydropyrazine[1,2:1,6]bis{(1S,R)-[1-dimethoxyethyl-2-yl]-2,3, 4,9-tetrahydro-1H-pyridino[3,4-b]indole}-1',4'-dione (3)
[0022] In 886mg (2.91mmol) 2, add 50mL of anhydrous DMF to dissolve, add 1.29g (3.4mmol) HATu, then use collidine to adjust the pH of the reaction solution to 8-9, after 24 hours of reaction, point the TLC plate to monitor the raw materials The point becomes shallower, and a new point is generated (CH 2 Cl 2 :CH 3 OH=60:1), the reaction was terminated; the reaction solution was concentrated under reduced pressure, dissolved in ethyl acetate, and successively washed with saturated NaHCO 3 solution, saturated NaCl solution, 5% KHSO 4 solution, saturated NaCl solution, 5% NaHCO 3 solution, washed with saturated NaCl solution for 3 times, combined the ethyl acetate layers, dried with anhydrous sodium sulfate for 2 h, filtered under reduced pressure, and concentrated the filtrate under reduced pressure to obtain a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com