Treatment of asthma with Anti-tslp antibody
An antibody and asthma technology, applied in the field of anti-TSLP antibody treatment of asthma, can solve the problems of patients with asthma who cannot control moderate to severe asthma
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
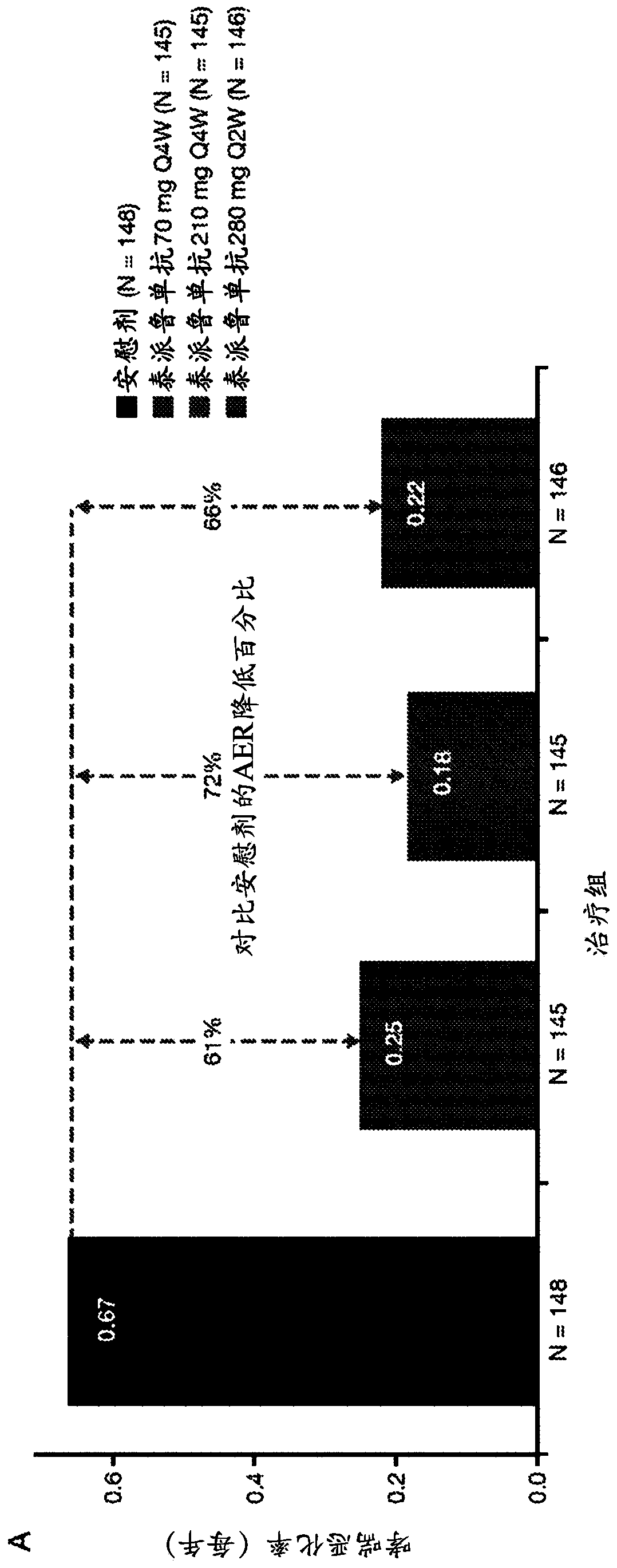
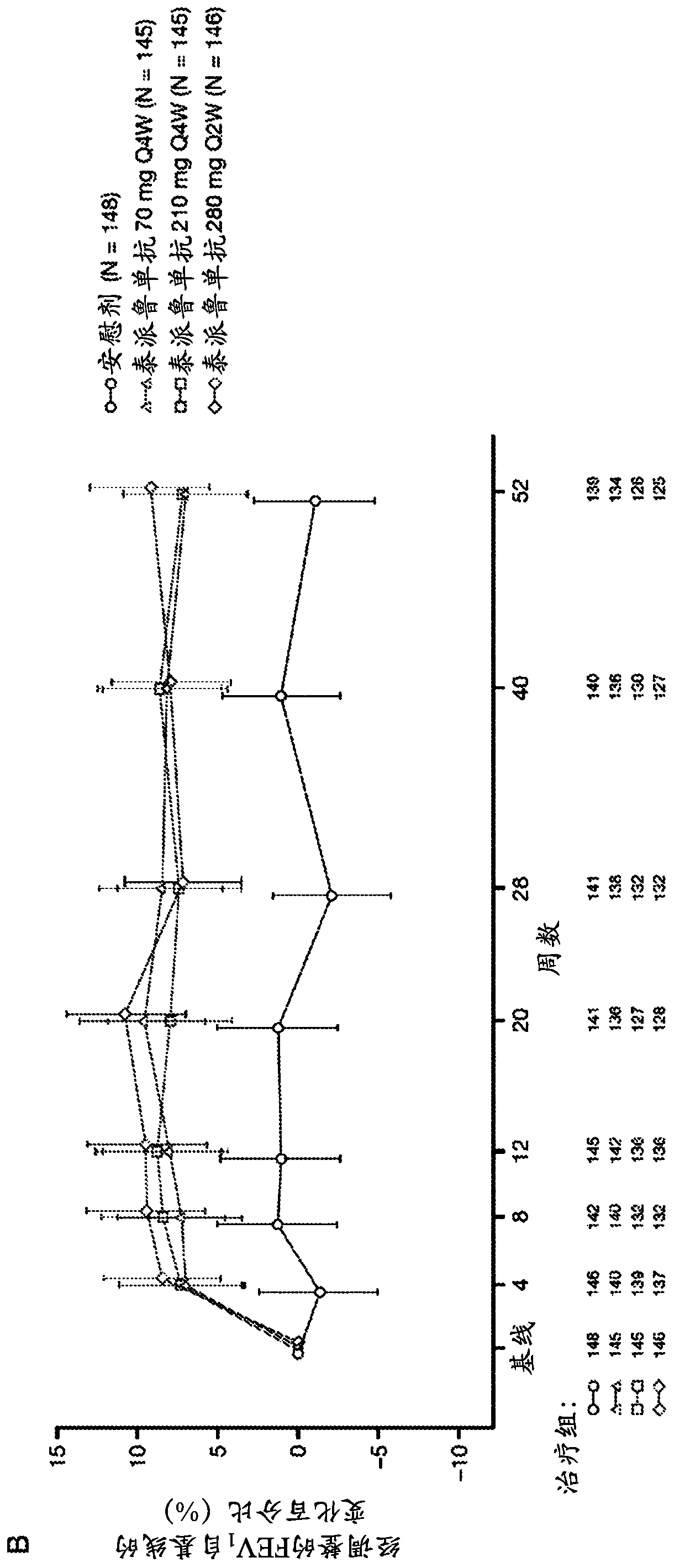
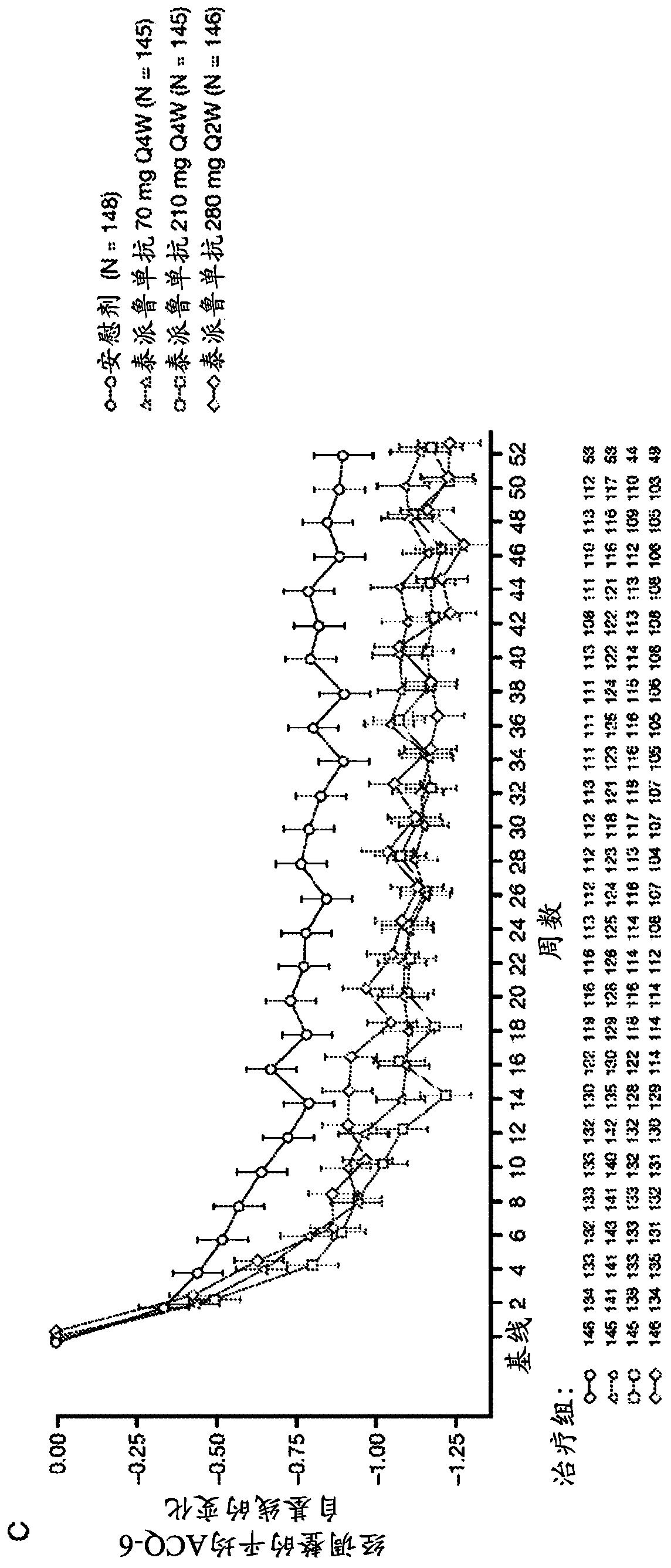
[0170] The anti-TSLP antibodies of the present invention are the first epithelial-targeted products that can have devastating efficacy in patients with non-eosinophilic and eosinophilic asthma. The high EOS group accounts for approximately 50%-70% of patients with severe asthma.
[0171] This example describes a multicentre, placebo-controlled, parallel-group, double-blind, phase 2 study conducted at 108 sites across 12 countries. Eligible patients were current never-smokers (≥6 months and with a history of 500 μg daily fluticasone or equivalent administered with the aid of a dry powder inhaler) combination therapy with inhaled corticosteroids for at least 6 months with poorly controlled asthma (according to defined severity GINA2012 Guidelines for Asthma 24). Patients were also required to have a history of at least two asthma exacerbations leading to systemic glucocorticoid therapy or one severe exacerbation leading to hospitalization within the 12 months prior to trial en...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com