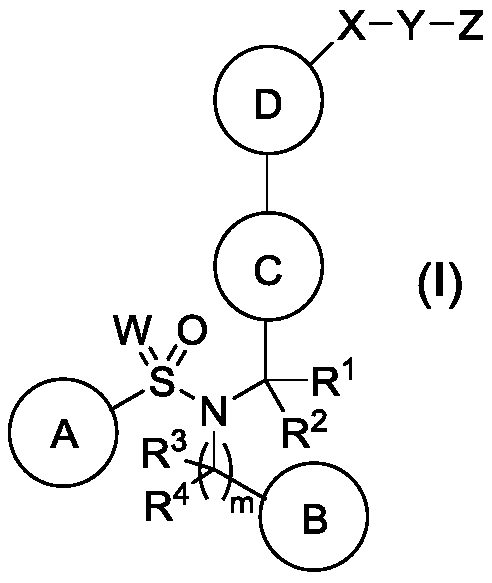
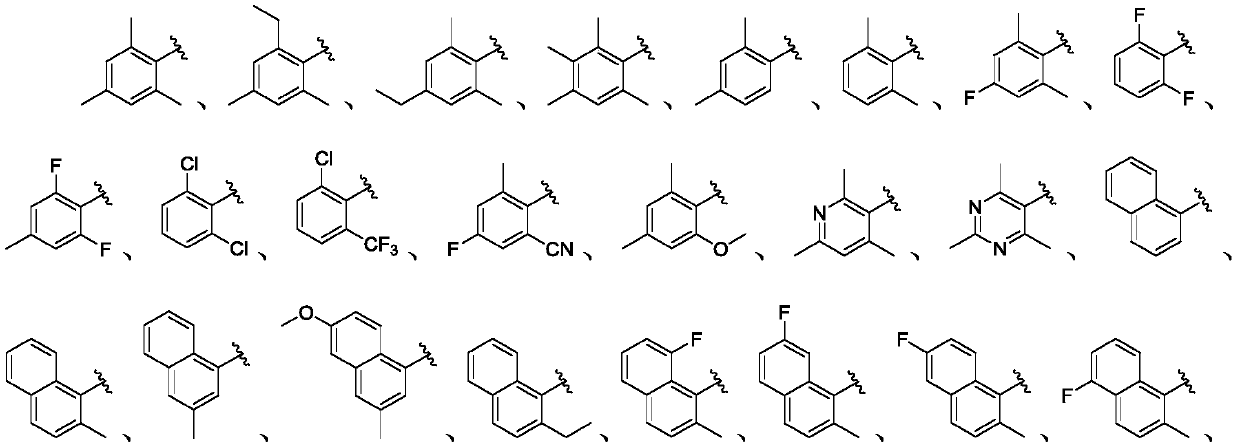
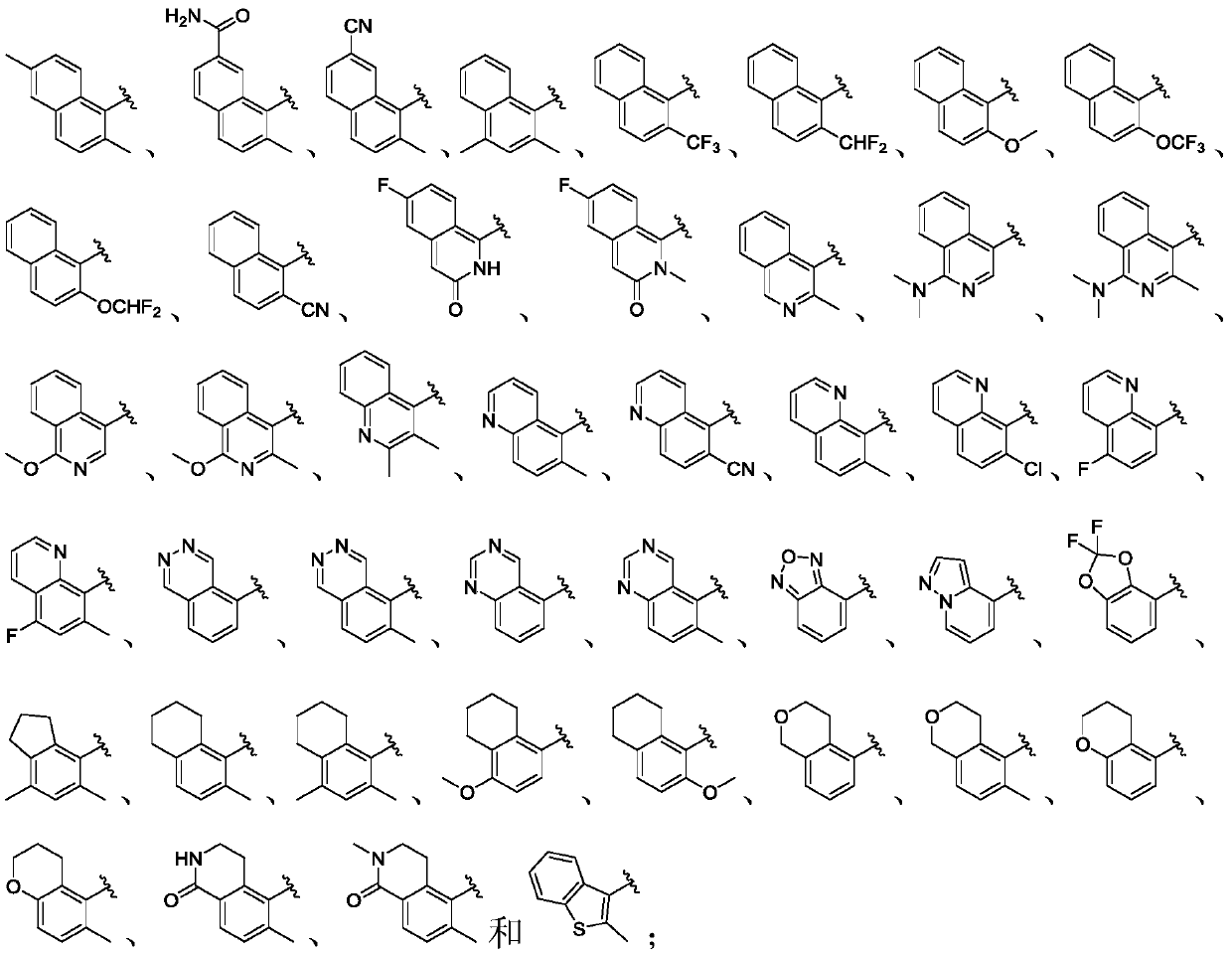
Liver x receptors (LXR) modulators
A solvate, enantiomer technology, applied in the field of regulation related diseases, can solve problems such as inactivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example P1
[0384]
[0385] Methyl 2-((3-bromophenyl)sulfonyl)propionate (P1)
[0386] Methyl 2-((3-bromophenyl)sulfonyl)acetate (500mg, 1.71mmol) and K 2 CO 3 (354 mg, 2.57 mmol) in acetone (20 mL) was added MeI (0.11 mL, 1.71 mmol). The reaction mixture was stirred overnight at 30 °C and filtered. The filtrate was concentrated to afford crude compound P1 as a yellow oil. MS: 307(M+1) + .
preparation example P2
[0388]
[0389] Methyl 2-((3-bromophenyl)sulfonyl)-2-methylpropanoate (P2)
[0390] A suspension of 2-((3-bromophenyl)sulfonyl)acetate (500 mg, 1.71 mmol) and NaH (152 mg, 60% on oil, 3.8 mmol) in anhydrous DMF (10 mL) was dissolved at 0 After stirring at °C for 0.5 h, MeI (0.7 mL, 3.77 mmol) was added to the solution at 0 °C. The mixture was stirred at room temperature for 2 h, washed with H 2 O diluted and extracted with EA (3x). The combined organic layers were washed with brine, washed with Na 2 SO 4 Drying and concentration afforded crude compound P2 as a yellow oil. MS: 321(M+1) + .
preparation example P3
[0392]
[0393] Step 1: tert-butyl 4-bromo-2,6-difluorobenzoate (P3a)
[0394]
[0395] 4-bromo-2,6-difluorobenzoic acid (25.0g, 110mmol), Boc 2 A mixture of O (50.0 g, 242 mmol) and 4-dimethylaminopyridine (1.3 g, 11 mmol) in tert-BuOH (200 mL) was stirred overnight at 40 °C, concentrated and passed through FCC (PE:EA=50:1 ) to obtain compound P3a as a yellow oil. MS: 292(M+1) + .
[0396] Step 2: tert-butyl 4-bromo-2-fluoro-6-((2-methoxy-2-oxyethyl)thio)benzoate (P3b)
[0397]
[0398] To a solution of methyl 2-thioglycolate (11.2 g, 106 mmol) in anhydrous DMF (50 mL) was added NaH (5.1 g, 60%, 127 mmol) at 0 °C. The mixture was stirred for 30 min. Then a solution of compound P3a (31 g, 106 mmol) in anhydrous DMF (100 mL) was added to the mixture. The mixture was stirred at room temperature for 2 h, washed with H 2 Diluted with O (1000 mL) and extracted with EA (3x). The combined organic layers were washed with H 2 O and brine washed, concentrated and p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com