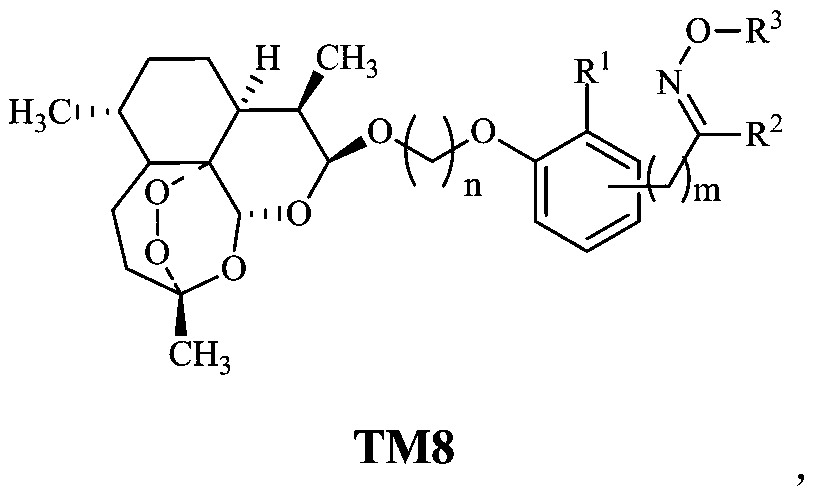
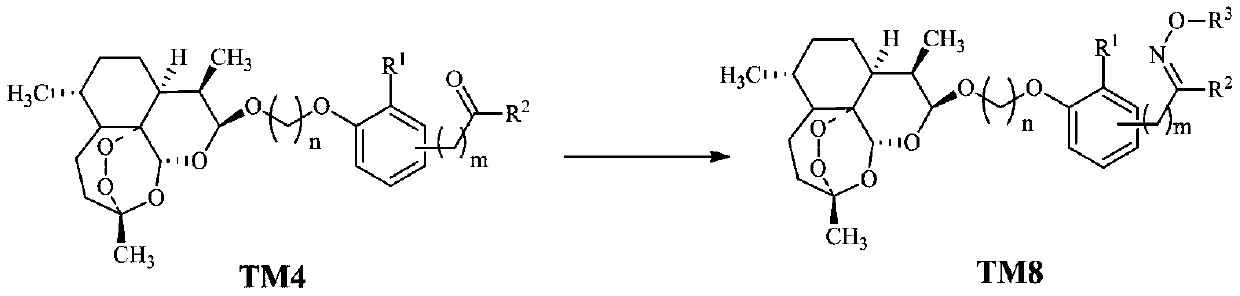
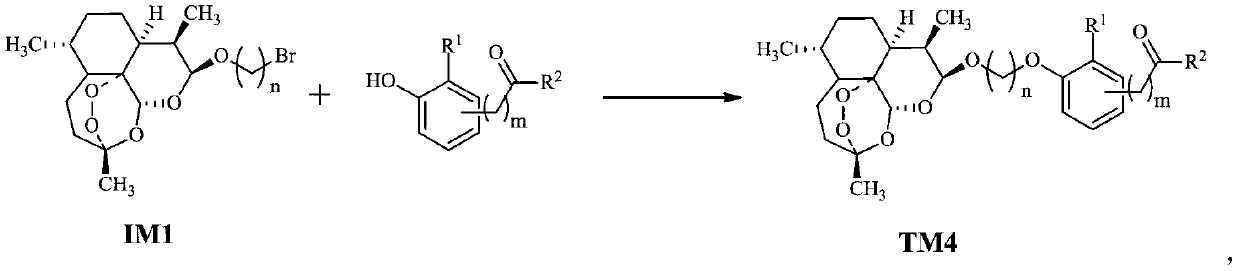
Dihydroartemisinin oxime-containing phenol derivatives as well as synthesis method and application thereof
A technology of dihydroartemisinin and synthesis method, which is applied in the field of chemical medicine, can solve problems such as unreported, achieve good application prospects, high synthesis yield, and the effect of inhibiting interleukin-17
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Embodiment 1, preparation of dihydroartemisinin-containing oxime phenol derivatives
[0030] (1) The preparation method of intermediate IM1 is as follows:
[0031]
[0032] Dihydroartemisinin (DHA), diethyl ether (Et 2 O) and bromoalcohol, add boron trifluoride-ether (BF 3 ·Et 2 O), under stirring reaction 5 ~ 20h, after the completion of the reaction, add saturated NaHCO 3 The reaction was terminated, the layers were left to stand, the aqueous layer was extracted with ethyl acetate (EtOAc or EA), the organic phases were combined, washed with saturated brine, anhydrous MgSO 4 Dry, filter with suction, and remove the solvent by rotating the filtrate under reduced pressure to obtain a crude product, which is recrystallized with petroleum ether (PE)-EA mixed solvent to obtain intermediate IM1.
[0033] (2) DHA and carbonyl-containing phenol conjugates, we use TM4 to represent in this application, and its preparation method is as follows:
[0034]
[0035] In a 1...
Embodiment 2
[0039] Example 2, Preparation of dihydroartemisinin-containing oxime phenol derivatives TM8-1~TM8-58
[0040] According to the preparation method described in Example 1 when n=2 or 3, R 1 H or -OCH 3 , R 2 H, -CH 3 or -CH 2 CH 3 , R 3 for H, CH 3 or CH 2 CO 2 H, m=0 or 2, a series of products TM8-1 to TM8-58 were prepared, and the respective reaction conditions, yields, product yields, and product melting points are shown in Table 1.
[0041] Table 1 Experimental results of synthesis of TM8 series compounds
[0042]
[0043]
[0044]
[0045]
Embodiment 3
[0046] Example 3, Test and Characterization of TM8-1~TM8-58
[0047] Carried out to embodiment 2TM8-1~TM8-58 series products 1 H NMR (AV-300), 13 C NMR (AV-75) and HRMS (Varian7.0T) testing and characterization, the data are as follows:
[0048]
[0049] TM8-1(E)-2-(3-(((3R,6R,8aS,9R,10S,12R,12aR)-3,6,9-Trimethyldecahydro-3H-3,12-epoxy[1,2] dioxepino [4,3-i]isochromen-10-yl)oxy)propoxy)benzaldehyde
[0050] 1 H NMR (300MHz, CDCl 3 )δ: 8.51(1H, s), 7.70(1H, d, J=6.6Hz), 7.32(1H, t, J=7.2Hz), 6.97-6.88(2H, m), 5.33(1H, s), 4.83(1H, d, J=3.3Hz), 4.15-4.08(3H, m), 3.59-3.52(1H, m), 2.65-2.60(1H, m), 2.39-2.28(1H, m), 2.13- 1.04(12H, m), 1.43(3H, s), 0.89(3H, d, J=7.5Hz), 0.86(3H, d, J=6.3Hz). 13 C NMR (75MHz, CDCl 3 )δ:156.95,146.36,131.15,126.88,120.85,111.90,104.19,101.90,87.84, 81.12,65.16,64.33,52.50,44.40,37.28,36.44,34.58,30.95,29.29,26.19,24.65,24.60,20.42, 13.0 .HRMScalcdforC 25 h 35 NO 7 [M+Na] + 484.2311, found 484.2310.
[0051] TM8-2(E)-3-(3-(((3R,6R,8...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com