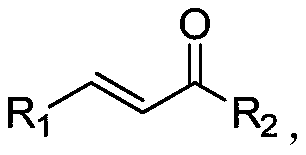
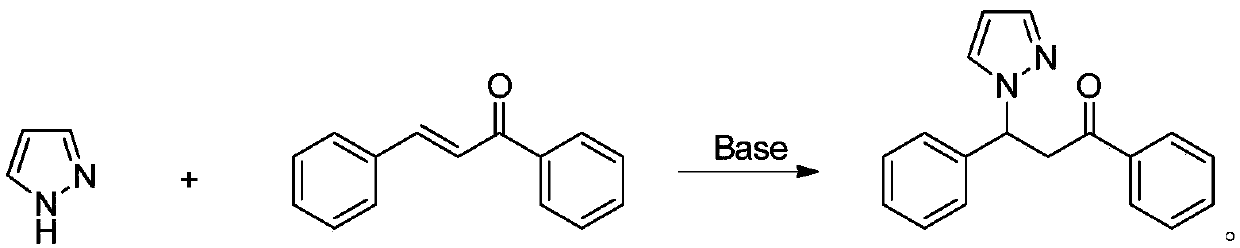
Method for synthesizing beta-(N-pyrazolyl) ketone compound through base catalysis
A ketone compound and pyrazole-based technology, which is applied in the field of base-catalyzed synthesis of β-ketone compounds, can solve the problems of poor universality of substrates, harsh reaction conditions, difficult separation, etc. Mild conditions and low dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026]
[0027] Add pyrazole (1.0mmol) and α,β-unsaturated ketone in turn to the reaction tube (1.0mmol), sodium tert-butoxide (0.02mmol), then add solvent toluene 2mL, react at room temperature for 6 hours, concentrate the reaction solution after the reaction, and separate by column chromatography to obtain the corresponding product with an isolated yield of 89%. 1 H NMR (400MHz, CDCl 3 )δ: 3.56(dd, J=5.2, 17.6Hz, 1H), 4.42(dd, J=8.4, 17.6Hz, 1H), 6.04(dd, J=5.2, 8.4Hz, 1H), 6.15(t, J =2.0Hz,1H),7.17~7.37(m,5H),7.41~7.42(m,2H),7.43~7.49(m,3H),7.89(d,J=7.2Hz,2H); HRMS(ESI) :calcd for C 18 h 16 N 2 O[M] + 276.1263, found 276.1276.
Embodiment 2
[0029]
[0030] Add pyrazole (1.1mmol) and α,β-unsaturated ketone in turn to the reaction tube (1.0mmol), sodium tert-butoxide (0.02mmol), then add solvent toluene 2mL, react at room temperature for 8 hours, concentrate the reaction solution after the reaction, and separate by column chromatography to obtain the corresponding product with an isolated yield of 92%. 1 H NMR (400MHz, CDCl 3 )δ: 3.64(dd, J=5.6, 17.6Hz, 1H), 4.43(dd, J=6.8, 17.6Hz, 1H), 6.08(dd, J=5.6, 6.8Hz, 1H), 6.23(s, 1H ), 7.28(d, J=1.6Hz, 4H), 7.43~7.55(m, 5H), 7.96(d, J=6.0Hz, 2H); HRMS(ESI):calcd forC 18 h 15 ClN 2 O[M] + 310.0873, found 310.0875.
Embodiment 3
[0032]
[0033] Add pyrazole (1.2mmol) and α,β-unsaturated ketone in sequence in the reaction tube (1.0mmol), potassium tert-butoxide (0.02mmol), then add 2mL of tetrahydrofuran as a solvent, and react at room temperature for 12 hours. After the reaction, the reaction solution is concentrated and separated by column chromatography to obtain the corresponding product with an isolated yield of 95%. 1 H NMR (400MHz, CDCl 3 )δ: 3.64(dd, J=2.8, 18.0Hz, 1H), 4.43(dd, J=8.0, 18.0Hz, 1H), 6.03~6.11(m, 1H), 6.22(d, J=9.2Hz, 1H ), 7.22(d, J=7.6Hz, 2H), 7.43~7.55(m, 7H), 7.96(d, J=7.2Hz, 2H); HRMS(ESI): calcd for C 18 h 15 BrN 2 O[M] + 354.0368, found 354.0369.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com