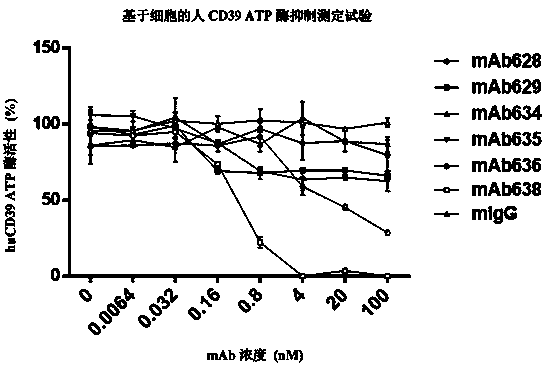
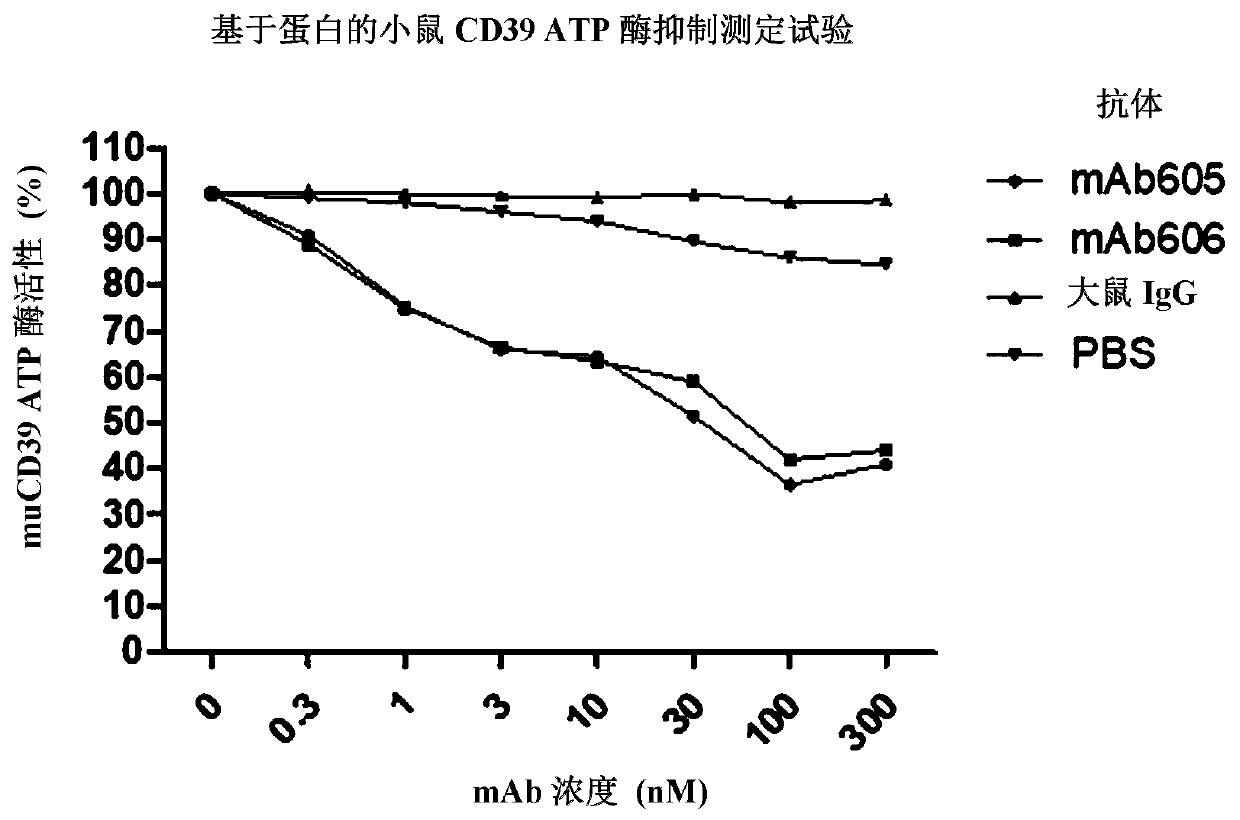
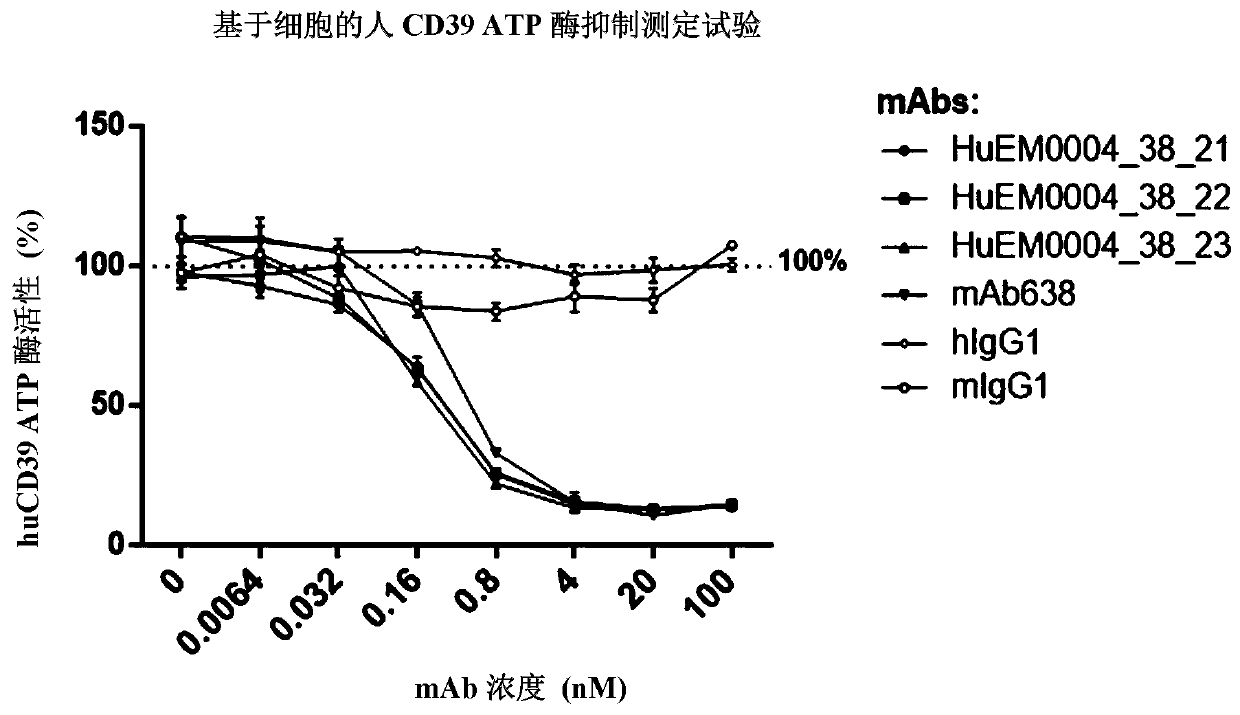
CD39 high-affinity antibody and use thereof
A CDR-H3, CDR-L3 technology, applied in the direction of antibodies, applications, anti-tumor drugs, etc., can solve the problem of loss of inhibitory function
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0133] Example 1: Production of anti-CD39 monoclonal antibodies
[0134] Mouse anti-human CD39 and rat anti-mouse CD39 monoclonal antibodies were obtained as follows.
Embodiment 11
[0135] Example 1.1(a): Immunization of mice with human CD39 antigen
[0136] 50 μg of recombinant purified human CD39 protein (R&D Systems, Inc., Minneapolis, MN, USA) mixed with complete Freund’s adjuvant, or 5×10 without adjuvant 6 Cells of the CHO-K1-human CD39 stable cell line were injected intraperitoneally on day 1 into two groups of five 6-8 week-old Balb / C and SJL mice. On days 14 and 35, 25 micrograms of recombinant purified human CD39 protein mixed with incomplete Freund's adjuvant, or 5×10 without adjuvant 6 The CHO-K1-human CD39 stable cell line was injected intraperitoneally into the same mice. A final boost with the same immunogen was performed 3-4 days prior to fusion.
Embodiment 11
[0137] Example 1.1(b): Immunization of rats with mouse CD39 antigen
[0138] On day 1, 100 μg of recombinant purified mouse CD39 protein (Chempartner Co., Ltd.; Shanghai) mixed with complete Freund's adjuvant was intraperitoneally injected into a 6-8 week old Sprague Dawley rat. On days 14 and 35, 50 μg of recombinant purified mouse CD39 protein mixed with incomplete Freund's adjuvant was injected intraperitoneally into the same rats. A final boost with the same immunogen was performed 3-4 days prior to fusion.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com