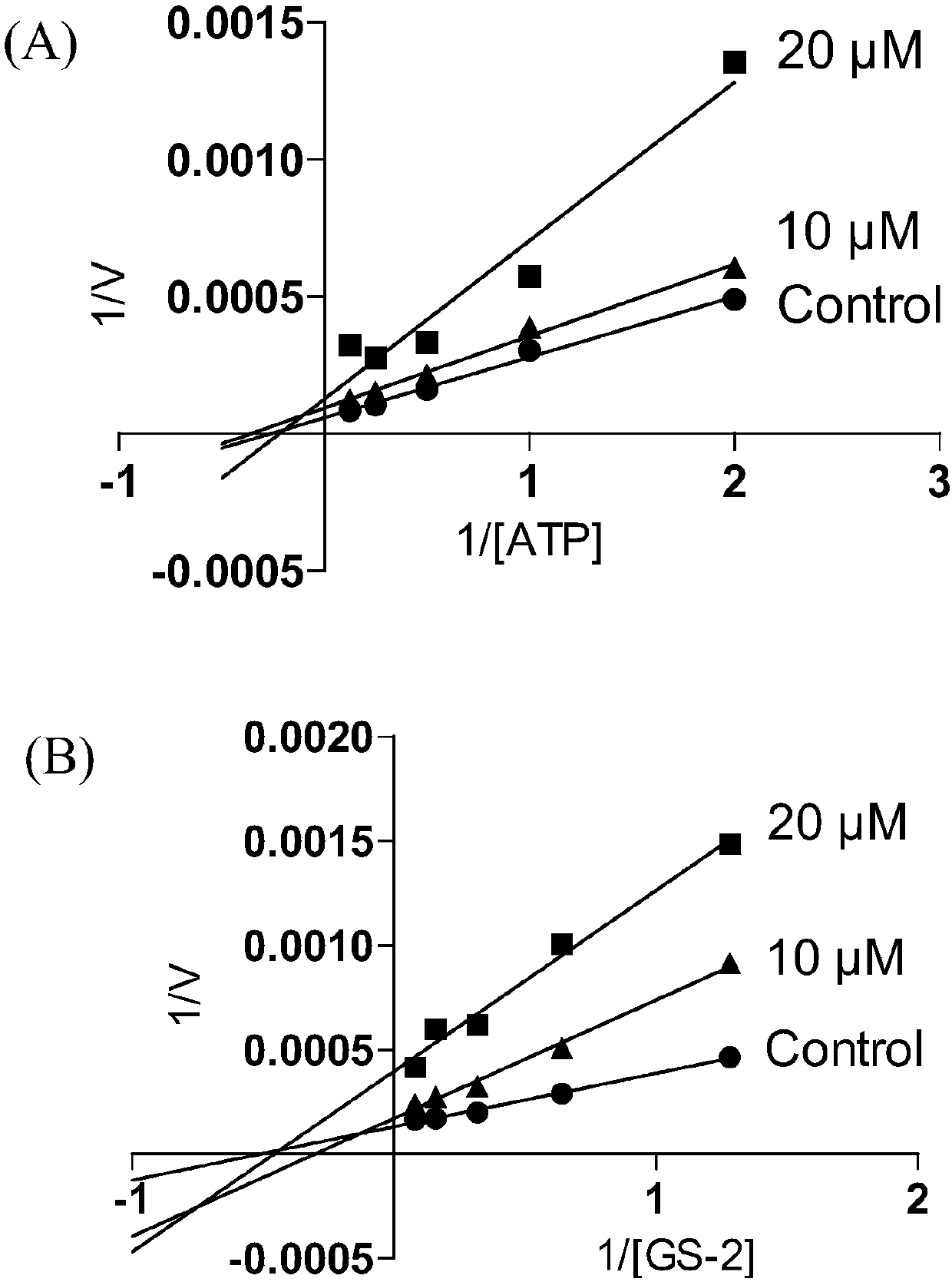
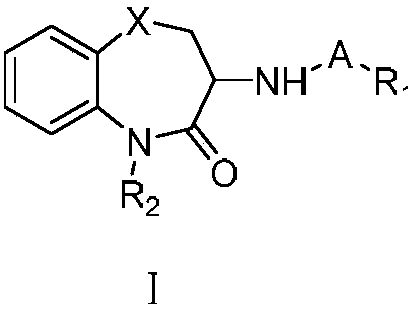
3-substituted-1,5-benzodiazepine compound and pharmaceutical application thereof
A technology of benzazepines and compounds, which is applied in the field of 3-substituted-1,5-benzazepinone compounds and their application in medicine, and can solve problems such as toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1: Preparation of (R)-N-(5-(2-nitrobenzyl)-4-oxo-2,3,4,5-tetrahydro-benzo[b][1,4]sulfur nitrogen miscellaneous -3-base) benzamide (7), its structural formula is as follows:
[0037]
[0038] Step 1: Preparation of N-Boc-L-cysteine (1)
[0039]
[0040] Add L-cysteine (6.0g, 50mmol), NaOH (1.2g, 50mmol), dioxane (30mL) and water (50mL) into the reaction flask, add (Boc) 2 O (17.0 g, 70 mmol), continue stirring at room temperature overnight. Dioxane was removed by concentration under reduced pressure, and the aqueous layer was extracted with ethyl acetate to remove unreacted Boc anhydride. The aqueous layer was adjusted to pH 1-2 with 1 mol / L hydrochloric acid, extracted twice with ethyl acetate, the combined organic phases were washed with saturated brine, dried over anhydrous sodium sulfate, and concentrated under reduced pressure to obtain 2.15 g of a colorless syrup (1 ), yield 97%. directly used in the next step reaction;
[0041] The second ...
Embodiment 2
[0059] Example 2: Preparation of methyl-(R)-4-((5-(2-nitrobenzyl)-4-oxo-2,3,4,5-tetrahydro-benzo[b][1, 4] Thiazepine -3-base) carbamoyl) benzoate (8), its structural formula is as follows:
[0060]
[0061] The preparation method was the same as that of compound 7 in Example 1, using monomethyl terephthalate instead of benzoic acid to obtain a white solid with a yield of 70%. 1 H NMR (400MHz, DMSO-d 6 )δ9.25-9.17(m,1H),8.07-8.00(m,3H),8.00-7.94(m,2H),7.79(d,J=8.2Hz,1H),7.68(d,J=7.8Hz ,2H),7.51(dd,J=13.7,8.0Hz,3H),7.33(d,J=9.1Hz,1H),5.45(d,J=17.3Hz,1H),5.31(d,J=17.3Hz , 1H), 4.67 (d, J = 9.7Hz, 1H), 3.86 (s, 3H), 3.62 (t, J = 9.3Hz, 1H).
Embodiment 3
[0062] Example 3: Preparation of (R)-4-methoxy-N-(5-(2-nitrobenzyl)-4-oxo-2,3,4,5-tetrahydro-benzo[b][ 1,4] Thiazepine -3-base) benzamide (9), its structural formula is as follows:
[0063]
[0064] The preparation method was the same as the preparation of compound 7 in Example 1, replacing benzoic acid with p-methoxybenzoic acid to obtain a white solid with a yield of 50%. 1 H NMR (400MHz, DMSO-d 6 )δ8.78(d, J=7.6Hz, 1H), 7.98-7.91(m, 1H), 7.85-7.70(m, 3H), 7.60(d, J=8.1Hz, 2H), 7.47-7.35(m ,3H),7.22(dt,J=9.1,4.7Hz,1H),6.97-6.86(m,2H),5.39(d,J=17.3Hz,1H),5.23(d,J=17.2Hz,1H) , 4.68-4.55 (m, 1H), 3.71 (s, 3H), 3.58-3.47 (m, 1H), 3.30 (d, J=11.4Hz, 1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com